Engineers from the Molecular Foundry and Energy Storage division have developed an electrolyte material for solid-state batteries that allows cathodes to retain up to 90% of their initial capacity upon reuse.
Many electric vehicles on the market today utilize lithium-ion batteries because of their impressive power-to-weight ratio. However, despite their suitability for such purposes, these batteries are heavy and challenging to recycle.
Engineers from the Molecular Foundry and the Energy Storage division at Lawrence Berkeley National Laboratory have crafted an electrolyte material for solid-state batteries. This advancement permits the cathodes to be reused with up to 90% of their original capacity.
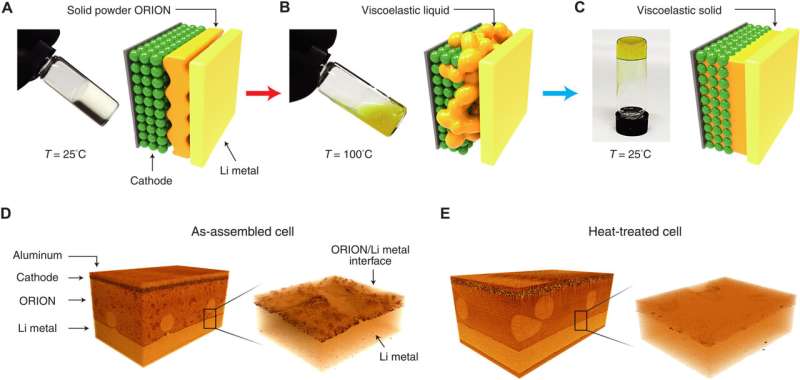
Lithium-ion batteries, true to their name, rely on lithium salt electrolytes. In the innovative approach, the team incorporated the same salt into their novel electrolyte but enhanced its properties by introducing organic-based zwitterionic polymers. They then assembled a battery using this modified electrolyte, pairing it with a lithium metal anode and cathodes composed of various materials, including lithium iron, phosphate, nickel, cobalt, and manganese. As indicated by the researchers, the battery produced is considerably more recyclable than existing batteries, irrespective of the chosen cathode type. This enhanced recyclability stems from the polymer in the electrolyte that facilitates its melting at 100°C.
After reaching the melted state, the battery can be conveniently deconstructed, enabling the individual components to be recycled. The researchers highlight that before reaching its melting point, the electrolyte remains highly conductive, viscoelastic and maintains a solid form at usual operating temperatures. Experiments on the battery revealed high conductivity at temperatures reaching 45°C. Additionally, the team observed that after disassembling the battery, the cathode retained 90% of its initial capacity, paving the way for straightforward recycling.
While further testing is required, the research group believes that the novel electrolyte will meet expectations. This could set the stage for future electric vehicles equipped with more eco-friendly batteries.
Reference: Jiwoong Bae et al, Closed-loop cathode recycling in solid-state batteries enabled by supramolecular electrolytes, Science Advances (2023). DOI: 10.1126/sciadv.adh9020






