As the global push for sustainable energy solutions intensifies, advancements in proton-conducting materials will play a critical role in developing efficient and reliable fuel cells and electrolysis cells.
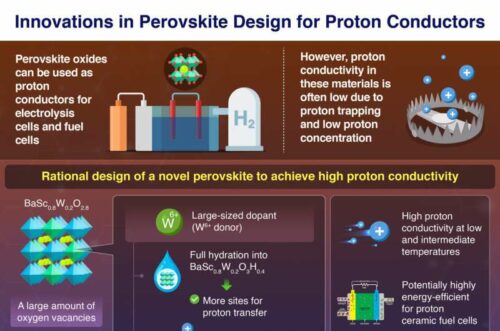
The researchers at Tokyo Institute of Technology (Tokyo Tech) have developed a perovskite material, BaSc0.8W0.2O2.8, which exhibits good proton conductivity at low and intermediate temperatures. Professor Masato Yashima and his team reported this advancement, which promises to significantly enhance the efficiency of protonic ceramic fuel cells (PCFCs) and proton-conducting electrolysis cells (PCECs).
The newly developed perovskite leverages a high concentration of intrinsic oxygen vacancies to achieve superior proton conduction. By incorporating large W6+ ions as dopants, the material can absorb more water, increasing proton concentration and mitigating proton trapping through electrostatic repulsion between the dopant and the protons.
Fuel cells, particularly those employing solid electrolytes, are emerging as crucial technologies for converting chemical energy from hydrogen or other fuels into electrical energy. Solid electrolytes provide inherent safety and stability advantages over liquid counterparts. Among these, PCFCs are of particular interest due to their operation with light protons (H+) rather than heavier oxide ions (O2−), allowing functionality at temperatures ranging from 50 to 500 °C. However, existing perovskite-based PCFCs struggle with low proton conductivity at these temperatures.
The Tokyo Tech team’s solution involves creating a perovskite with significant oxygen deficiencies, specifically BaSc0.8W0.2O2.8, which benefits from increased proton concentration. The introduction of W6+ cations results in a larger lattice volume, enhancing water uptake and further boosting proton conductivity. The high positive charge of W6+ ions reduces proton trapping, as evidenced by ab initio molecular dynamics simulations that illustrate the repulsion effect on proton migration.
This research not only addresses the limitations of current proton conductors but also lays the groundwork for the rational design of high-performance materials for PCFCs and PCECs. “The stabilization of perovskites with disordered intrinsic oxygen vacancies and full hydration enabled by doping of large donor dopants could be an effective strategy towards next-generation proton conductors,” explains Yashima.