The electro-biodiesel process transforms CO2 into fuel, offering 45 times the efficiency and drastically less land use than traditional methods.
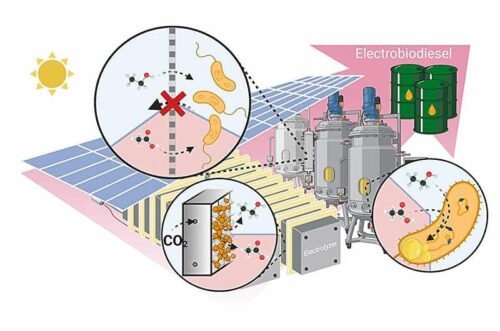
Researchers at the University of Missouri, have collaborated with researchers from Texas A&M University. Their joint research involved the electrocatalysis of carbon dioxide to produce an electro-biodiesel. This new form of biodiesel is 45 times more efficient and requires 45 times less land compared to traditional soybean-based biodiesel.
The team explained that they have utilized electrocatalysis. This process involves initiating a chemical reaction through the transfer of electrons to and from reactants on catalyst surfaces, transforming carbon dioxide into biocompatible intermediates like acetate and ethanol. These intermediates were then processed by microbes into lipids, or fatty acids, which were subsequently used as biodiesel feedstock.
The research teams have developed a microbial and catalyst process that enabled their electro-biodiesel to achieve a solar-to-molecule efficiency of 4.5% for converting carbon dioxide into lipids. This efficiency is significantly higher than that of traditional biodiesel. They noted that in comparison, natural photosynthesis in land plants typically has an efficiency below 1%, where less than 1% of sunlight energy is converted into plant biomass through the transformation of CO2 into various molecules that facilitate plant growth.
The team developed a novel zinc- and copper-based catalyst to initiate electrocatalysis, producing diatomic carbon intermediates that were transformed into lipids using an engineered strain of the Rhodococcus jostiii (RHA1) bacterium, known for its high lipid content. This strain also enhanced the metabolic processing of ethanol, aiding in the conversion of acetate, an intermediate, into fatty acids.
Upon refining this process, the team evaluated its environmental impact, discovering promising results. The use of renewable resources for electrocatalysis in the electro-biodiesel process resulted in a reduction of 1.57 grams of carbon dioxide per gram of electro-biodiesel produced, factoring in by-products like biomass and ethylene. This suggests the potential for negative emissions. By comparison, traditional diesel production from petroleum emits 0.52 grams of carbon dioxide per gram, while other biodiesel production methods emit between 2.5 and 9.9 grams of carbon dioxide per gram of lipids produced.












