High-quality medical services are critical all over the world. India’s medical communities are working hard to bring the best infrastructure and most upgraded setups to us. Quality and scope of services of electromedical devices have been expanded—more so to intensive care units (ICUs). Coeo Labs, a Bengaluru-based startup, designs and delivers such advanced equipment.
Problems in ICUs
Coeo Labs had an eye to solve the existing challenges in ICUs—challenges that affect patients and staff alike. Co-founder, Coeo Labs, Nitesh Kumar Jangir says, “Annually, there are close to 50 million patients in ICUs globally. Out of these, 19 million get on long-term ventilation (over 48 hours). During this time, they are prone to multiple hospital-acquired infections. It is estimated that 31 per cent of these patients develop ventilator-associated pneumonia (VAP) during their stay in the ICU, annually.”
VAP has an annual mortality rate of about 42 per cent. Moreover, it creates collateral complications, both for patients as well as the medical organisation(s). Jangir continues, “When a patient develops VAP, the stay in the ICU typically increases by five to seven days. This results in excess utilisation of 30 million ICU bed-days, and results in an additional financial burden of more than about INR 400,000 for each patient in emerging economies (like India).”
Jangir along with Nachiket Deval (co-founder) attended Affordable Innovation Fellowship in healthcare from InnAccel. Here, they identified and analysed the highest unmet clinical needs in the field of emergency and critical care. Among the 120 clinical needs that were identified, preventing VAP came at the top.
The main route of VAP in a patient is an endotracheal tube that is inserted into the trachea for breathing purposes, while she or he is sedated and, essentially, in an immuno-suppressed condition. The tube may turn into an entry channel for infectious microbes, further aided by oral and nasal secretions that collect in specific places in the respiratory tract. This leads to inflammation of the lungs, resulting in VAP.
Jangir points out, “Currently, manual suctioning is done by nurses and that too only in the oral cavity of the ventilated patient, which is inadequate to prevent VAP. Insufficient hand hygiene by the caregiver, non-adherence to clinical protocols and lack of trained ICU staff are some other factors for cross-infection.”
VAPCare: what it does and how it works
Coeo Labs developed an artificial intelligence (AI)-based secretion management and oral hygiene system for ventilated patients, called VAPCare. It provides targeted automated suctioning of secretions, minimising the chances of contracting VAP and enabling to eliminate the need for constant physical interaction between caregivers and patients to prevent cross-infection.
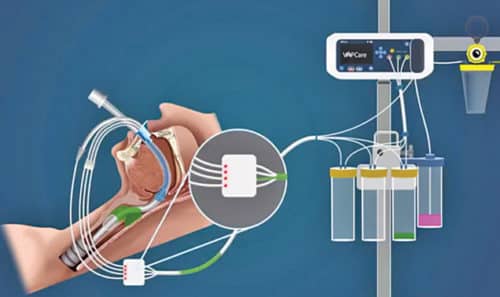
The device constitutes a disposable lumen that rides over an existing endotracheal tube. It has three suction ports, one each in oral, oropharyngeal and sub-glottic regions. The lumen gets connected to an electronic base of VAPCare and controls the suction based on sensor input. An electronic timer and sensor-based suctioning detect the presence of secretions in the suction catheter. The device continues suctioning until it finds no secretion in the suction catheter. Suction pressure is also controlled electronically.
This machine can also be used for oral lavage using an antibacterial solution through disposable lumen based on user-defined timing to maintain oral hygiene. Starting cost of one VAPCare unit is INR 350,000.
Coeo Labs received European CE certification and was granted a patent in multiple countries, including the US and China for VAPCare.
Challenges in development
The manufacturing ecosystem put a lot of obstacles before Coeo Labs. The company faced the biggest challenge in finding quality Indian manufacturers to support it for small batch manufacturing. Finding reliable manufacturers on specialised material was difficult, too.
Jangir adds, “Electromagnetic interference (EMI), electromagnetic compatibility (EMC) and electrical safety testing according to IEC 60601-1 is expensive and unaffordable for startups.”
Beside engineering challenges, there were clinical challenges, such as initial lack of clear guidelines for clinical trials of a new product. However, Jangir says that new rules have clarified this aspect.
Target group and roadmap ahead
VAPCare is mainly targeted at ICUs of hospitals and medical facilities, both private and public, of all scales, including small hospitals. Besides reducing VAP infection and improving overall medical care, it also reduces cost on staff, bed occupancy due to improved healthcare, collateral cost for hospitals on facilities as well as patients on bills.
The product was launched in India on August 1, 2018. The team is planning to deploy it across the nation this year. Going forward, it will further expand sales in Europe and launch VAPCare in the US.
It is also set to launch a new product—called Saans—which is a low-cost neonatal breathing support for short-term use in low-resource setting (like in ambulances), by the end of this year. “We have a product pipeline to create more solutions and save lives,” Jangir remarks.










