Breath Analyzers are used to descry and quantify the alcohol content in the subject’s breath non-invasively. These are invented by Robert Frank Borkenstein. These devices generate an estimated Blood Alcohol Concentration (BAC) in some acceptable units. Although the observed air/breath has small amount of alcohol concentration, though the results are relatively true to the actual values.
Evaporation of alcohol from the circulating blood to the lungs air is the basis of Breath Analyzer devices. After absorption of alcohol by the digestive organs, it enters the blood stream and travels through the body. During the breathing process, the oxygen from the lungs air enters into the blood and carbon-dioxide from the blood stream evaporates into the breath. When a person is drunk, in addition to carbon-dioxide, a modest quantity of alcohol also gets released into the breath through gaseous exchange process. As per The Henry’s law, the quantity of alcohol available in the person’s breath depends on its concentration in the blood stream. In equilibrium, the ratio of concentration of alcohol in blood (Blood Alcohol Concentration-BAC) to that in breath (Breath Alcohol Concentration-BrAC) is 2300:1 and it is almost constant. Statutory BAC limits are reported in concentration units of mg/100 ml, mg/g, g/l or mg/ml and the corresponding BrAC limits are reported as mg/l, g/l and g/100 ml, depending on the country.
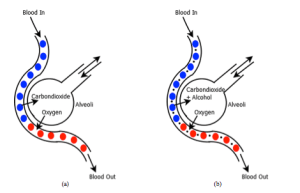
To get a reliable BAC measurement, the sampled breath must be nearest to the blood vessel. Figure 1 shows the gaseous exchange profile of a normal person and a drunken person. In lungs there present a small deep capillaries viz. alveoli which are closest to the blood vessels by a thin membrane. Carbon-dioxide from the blood vessels along with alcohol content is exhaled through the alveoli.
Breath alcohol concentration measures how intoxicated a person is at a given time. The results can fluctuate even within the course of a day. The more alcohol a person consumes, the higher his BAC should be. If he stops consuming alcohol, his BAC may still continue to rise because some of the alcohol consumed earlier may not have been absorbed into the bloodstream at the time of the initial testing. Eventually, if consumption is discontinued, the level of alcohol in the blood should begin to drop, resulting in a lower BAC.
The ongoing demand to create a device for Breath Analysis which should be portable, accurate, simple to operate & calibrate, provide data safety and integrable with modern smartphones prompted STMicroelectroincs to develop “Breath Analyzer” using embedded technology. The design is compact and easy to calibrate at user level using NFC link. The high quality solenoid air-pump along with Fuel-Cell sensor makes these devices veracious.
Solenoid Air Pump
Solenoid air pump is used to collect the sample, then throwing it on-to the sensor wafer (Fuel-Cell). The sensor is then activated for a predefined interval; say 200 mS. The collected sample is then blown-out through solenoid air-pump to reset the sensor wafer so that the new sample could be collected. This way, the procedure is triparted – Sampling; Holding and Reset. The solenoid air-pump area is directly proportional to the sample volume. The typical values of solenoid diameter and sample volume are given in the Table-1 below:
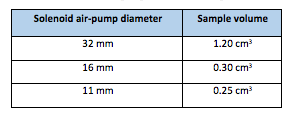
The sample area can be decimated in tune with the sample volume without compromising the sensor reading accuracy. A sample volume in the range of 0.25 cm3 to 0.50 cm3 is sufficient for precise measurement. The smaller diameter of Solenoid air pump is preferable, however, it burdens the associated electronics as the signal amplitude reduces which in turn reduces SNR (signal-to-noise ratio).
Breath Analyzer Sensors
The sampling of breath is done by Breath Analyzer sensor. Various types of these sensors are available viz. fuel-cell sensor, semiconductor sensor and spectrophotometer sensor each having some advantages and limitations over the others.
Electrochemical fuel cell breath analyzers are devices in which an electrical current is produced as a result of a chemical reaction taking place on the surface of an electrode system. In Fuel cell sensors alcohol (ethanol), undergoes a chemical oxidation reaction at a catalytic electrode surface (platinum/gold) to generate a quantitative electrical response. These sensors are highly specific and sensitive to alcohol and measurement cannot be influenced by endogenous substances such as acetone (produced by diabetics), Carbon Monoxide or Toluene. They have high calibration stability. These have an average life span of 5 years. These sensors cannot detect if breath sample is alveolar (deep lung air). As a result it may produce a falsely high reading if a subject has recently drank and still has alcohol in his mouth (highly unlikely as mouth alcohol evaporates very quickly).
Semiconductor sensors offer an affordable means to measure their breath alcohol with some compromises on reliability and accuracy. Semiconductor technology uses an oxide sensor to measure the reactivity between the tin dioxide (SnO2) in the sensor and the ethanol molecules in the breath sample. When the ethanol molecules come in contact with the tin dioxide the reaction changes the electrical resistance of the sensor. The semiconductor measures this difference and calculates an estimate of the BAC of the sample. These sensors are of affordable cost because of lower cost of manufacturing and supports low power portable systems. On the flip side, these are unstable and highly sensitive to the atmosphere, altitude and elevation.
Spectrophotometer technology is used in large, table-top breath analyzers. Spectrophotometers work by identifying molecules based on the way they absorb infrared light. The level of ethanol in a sample is singled out and measured, and a subject’s alcohol level can then be determined. These devices are expensive and are normally available on request.
Considering the higher calibration stability, longer life span and higher accuracy, electrochemical fuel cell sensors are used in STMicroelectronics “Breath Analyzer” design.
ST Microelectronics Breath Analyzer Architecture
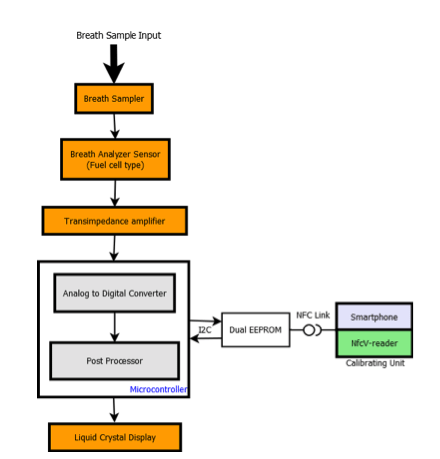
Breath Analyzer hardware architecture as shown in Figure 2 is a portable battery operated design based on 16 MHz proprietary STM8L core. STM8L includes an integrated debug module with a hardware interface (SWIM – Single wire interface module) which allows non-intrusive In-Application debugging and ultra-fast Flash programming. The ultralow power STM8L152R8T6 operates from a 1.8 V to 3.6 V power supply. A comprehensive set of power-saving modes allows the design of low-power applications.
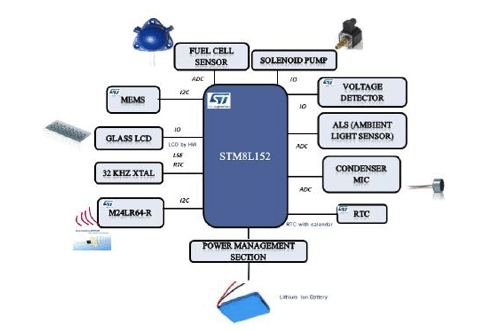
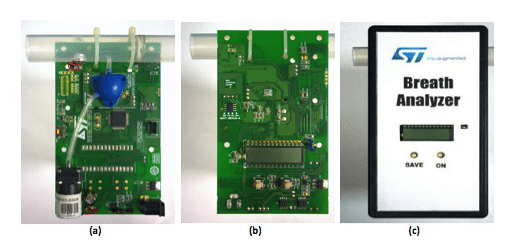
Breath analyzer system reference design is shown in Figure 3. The front and back panels of the reference design PCB are shown in Figure 3(a) and Figure 3(b) while Figure 3(c) depicts the packaged product. It is powered by 3.7V Lithium ion battery which can be charged by a battery charging IC STC4054GR using a wall adapter and low battery is indicated using a voltage detector IC STM1061N31WX6F. LED backlight for LCD display is switched on, automatically by sensing the ambient light intensity using ALS (Ambient Light Sensor). A condenser microphone is used to detect if a person has blown into the mouthpiece in order to actuate the pump and collect a precise, fixed volume (0.25ml) of air sample. Air sample containing alcohol interacts with fuel cell sensor which produces current proportional to alcohol concentration.
The current produced by the sensor is converted into voltage using op-amp TS507ILT configured as a trans-impedance amplifier. Integrated 12-bit ADC of STM8L is used to sample this voltage proportional to the sensor current and integrated over time. The resulting output is converted into BAC using first order linear equation coefficients derived during calculation. This information is displayed on LCD and user can optionally save this reading into Dual interface EEPROM.
Key Features
- STM8L low power MCU based reference design to measure the Blood Alcohol Concentration (BAC) non-invasively.
- Fuel cell sensor is used because of its greater specificity as compared to semiconductor sensors. This is especially important in reducing false positives due to acetone, which can occur in substantial qualities in the breath of diabetics.
- Current Integration Method of fuel cell output is used to determine the BAC. This method gives very stable short term and long term calibration stability.
- A rechargeable 3.7V Lithium Ion battery is used to power the system.
- Low battery detection is implemented using STM1061N31 voltage detector designed specifically for single cell Li-ion based solutions.
- Glass LCD with 6 alphanumeric characters to display information.
- Automatic LCD backlight is implemented using ALS to make the LCD screen visible in darkness.
- Can save up to 396 BAC readings along with date and time stamp in an on-board dual interface EEPROM.
- Android Application for data logging with RF EEPROM over NFC Interface.
- On board battery charging circuit based on STC4054GR powered using external 5V wall adapter.
- Ultra low standby power consumption in the order of 8uA including timekeeping operations by virtue of efficient hardware and firmware design.
Typical Applications
- Breath Analyzers are being used by Law enforcement.
- It is used as an Ignition Interlock device in vehicles.
- It can also be used handy by individuals to ensure that they can drive legally and safely.
Response curves
Normally two methods viz. Peak voltage method and Current Integration methods are used to calculate the final BAC values.
In case of current integration method, the readings from the Breath Analyzer Sensor are captured and then converted to an equivalent voltage through some associated circuitry. The voltage so obtained is directly proportional to the electric current input from the sensor. The graph of the captured signal is shown in Figure 4 for normal as well as drunken person.
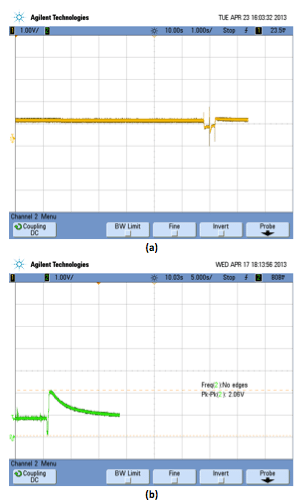
A current threshold is defined to fix the minimum resolution and the values above that threshold signify the alcohol content of a drunken person. A time window is defined to capture the breath through the Breath Analyzer sensor. All the values above the current threshold are integrated and averaged within that time interval to get the final BAC values.
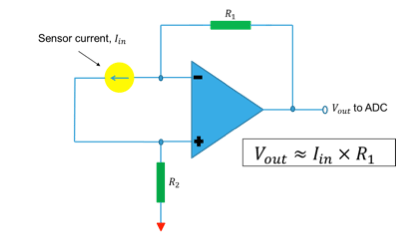
On the other hand in case of peak voltage method, the current output from the Breath Analyzer sensor is fed to an associated circuitry to convert to an equivalent voltage as shown in Figure 5. The output voltage peaks are then processed by the associated microcontroller unit and then internal ADC inside microcontroller is actuated and finally ADC output is post-processed to quantify the BAC.
Chemical equations
In case of fuel cell sensor (used in the STMicroelectronics solution), two terminals viz. anode and cathode are available. When a drunken people breath is sampled, the alcohol content in its breath is sensed by anode terminal and converted to ethanoic acid a.k.a. acetic acid.
CH_3 CH_2 OH(Alcohol)+ H_2 O(Water)→CH_3 COOH(Ethanoic acid)+4H^++4e^-
Also at the cathode terminal, the oxygen present in the atmosphere is reduced to water.
O_2 (Atmospheric oxygen)+ 4H^++4e^-→ 2H_2 O(Water)
As a whole chemical system, the products are ethanoic acid and water as shown in the equation below:
CH_3 CH_2 OH(Alcohol)+O_2 (Atmospheric oxygen) →CH_3 COOH(Ethanoic acid)+H_2 O(Water)
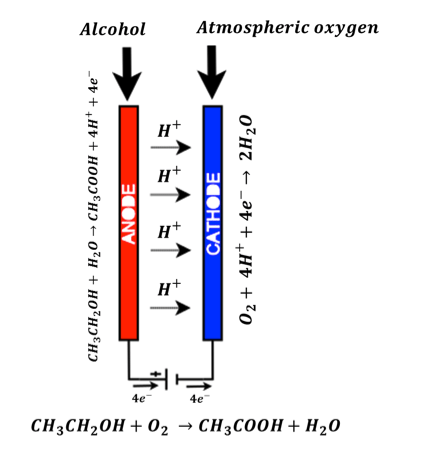
Figure 6 shows the fuel cell based Breath Analyzer sensor chemical reactions and the current flow mechanism. The electric current is produced because of flow of electrons between anode and cathode as shown in the above equation. This current is proportional to the concentration of alcohol content at the anode terminal. It is captured by the microcontroller and post processed to get the BAC value on to the LCD display.
Device Calibration
Fuel cell based Breath Analyzer sensors are susceptible to errors due to anode/cathode corrosion/infection by the dirt and other chemical reactions in addition to ethanol. This requires the filth removal and calibration which is normally done once a year.
Various methods of calibrating these sensors are available viz. dry and wet processes. The dry method involves passing the gaseous mixture of nitrogen and ethanol through the fuel cell filaments and then calibrating the sensor using hand-held device. This method requires less cost however accuracy available is also less. In case of wet method, the fuel cell filaments are soaked into ethanol and water solution and then system is simulated for calibration. This calibration strategy is expensive and requires bulky instruments; however, the higher calibration accuracy could be achieved.
The BAC value measured by the Breath Analyzer device is given by,
BAC %= (ADC value)/(Threshold)
Here ADC value is the value obtained after current integration and Threshold is the reference threshold to achieve the correct BAC%. During calibration, Threshold is normally changed to achieve the corrected results. Default Threshold in the demo board developed on STMicroelectronics’s STM8L device is 0x5000. If the Breath Analyzer sensor is exposed to some sample solution/mixture for which BAC% is previously known, the Threshold could be adjusted such that the output BAC% from the Breath Analyzer device matches exactly with the known BAC% value.
An Android based application, NfcV-reader, has been developed by STMicroelectronics to communicate with NFC enabled smartphones and change the Threshold value of Breath Analyzer device. This application can be directly downloaded from the Google Play Store.
To change the Threshold value, place a NFC enabled smartphone at defined location as shown in Figure 7 and Figure 8 and write the desired Threshold value (in hexadecimal) at 0x0000 location. After updating the value the device shall take the new Threshold value for BAC% calculations.
Conclusions
Convenient monitoring of Breath Alcohol content and in turn Blood Alcohol content is necessary for real-time applications viz. law enforcement, ignition interlock and self-assessment for safe driving. This can be made possible by portable machines designed specifically for such monitoring. The Breath Analyzer reference design developed by STMicroelectronics is low power (require rechargeable 3.7V Lithium Ion battery) and requires ultra-low standby power consumption. The sensor used in this design is of fuel cell type and STM8L microcontroller unit is used for data processing. In addition, STMicroelectronics free Android App ‘NfcV-reader’ provides quick calibration of the device. The present solution could be inchoatus for modern policing and law control.
Authors
· Salil Jain, Sr. Design Engineer, IPD System Lab and Technical Marketing, STMicroelectronics, India
· Alok Mittal, Group Manager, IPD System Lab and Technical Marketing, STMicroelectronics, India
· Saurabh Sona, Technical Leader, IPD System Lab and Technical Marketing, STMicroelectronics, India













Interesting article…