Modern battery cells use a variety of chemicals to power their reactions. Common battery chemistries include:
Zinc-carbon. These are the lowest- cost primary (non-rechargeable) cells meant for household use. The anode is zinc, the cathode is manganese dioxide and the electrolyte is ammonium chloride or zinc chloride. These cells produce very low power, but have a good shelf life and are well suited for clocks and remote controls.
Alkaline. These are the most commonly used primary cells. The cathode is made of manganese dioxide, while the anode is zinc powder. These cells have derived their name from the potassium hydroxide electrolyte, which is an alkaline substance. These provide more power-per-use than zinc-carbon and secondary (rechargeable) battery cells, and have an excellent shelf life.
Lithium. These are primary cells with a lithium metal or a lithium compound as an anode. These offer performance advantages well beyond the capabilities of conventional aqueous electrolyte battery systems. Their shelf life can be well above 10 years and they work at very low temperatures. These are mainly used in small formats (coin cells up to AA size) because bigger sizes of lithium batteries are a safety concern in consumer applications and are only used in military applications.

Silver oxide. These are also primary batteries with relatively very high energy/weight ratio. Their cost is linked to the price of silver. These are available in either very small sizes as button cells, where the amount of silver used is small and not a significant contributor to the overall product cost, or in large custom-design batteries, where the superior performance characteristics of the silver oxide chemistry outweigh cost considerations.
Nickel-cadmium. These are rechargeable or secondary batteries which use nickel oxide hydroxide and metallic cadmium as electrodes. The abbreviation Ni-Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd). Ni-Cd batteries are rugged and reliable. These exhibit high-power capability, wide operating temperature range and long lifecycle, but have low runtime per charge.
Nickel-metal hydride. A nickel–metal hydride battery cell, abbreviated as NiMH or Ni–MH, is also a rechargeable cell. It uses positive electrodes of nickel oxyhydroxide (NiOOH), such as the NiCd, but the negative electrodes use a hydrogen-absorbing alloy instead of cadmium, being in essence a practical application of nickel–hydrogen battery chemistry. An NiMH cell can have two to three times the capacity of an equivalent-sized NiCd, and its energy density approaches that of a lithium-ion cell.
Lithium ion. A lithium-ion cell (sometimes called Li-ion battery) is a rechargeable battery cell in which lithium ions move from the negative electrodes to the positive electrodes during discharge and back when charging. These batteries use an intercalated lithium compound as the electrode material, compared to the metallic lithium used in non-rechargeable lithium batteries.
Lead-acid. These are the most popular rechargeable battery cells worldwide. Both the battery product and the manufacturing process are proven, economical and reliable. However, as these batteries are heavy and use a liquid electrolyte, they are not used in portable consumer electronics.
Technical standards for battery sizes and types are published by standards organisations such as International Electrotechnical Commission (IEC) and American National Standards Institute (ANSI). Some sizes of batteries are D, C, AA, AAA, AAAA, A23, 9V and CR2032.
Accelerated testing
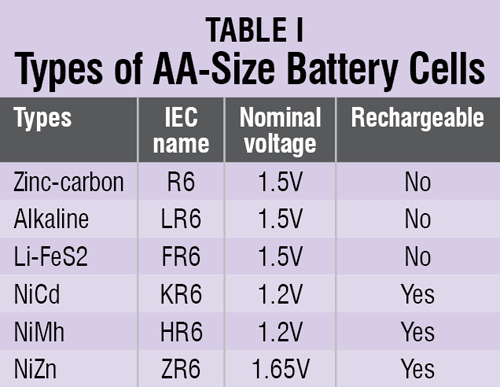
Different AA-size primary battery cells were chosen for testing in EFY lab. These cells come with a variety of chemistries and therefore the difference in their prices. You can identify different cells with their IEC names as shown in Table I.
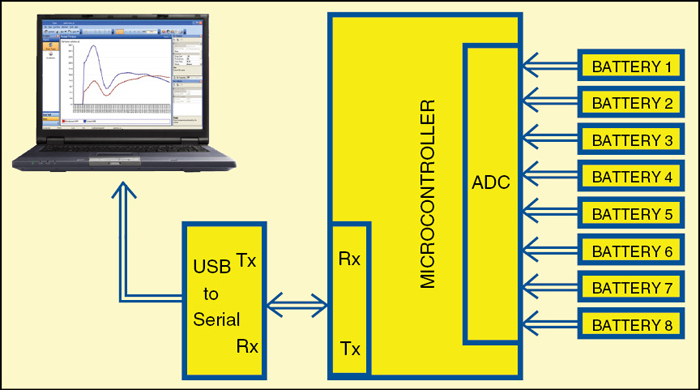
Fig. 2 shows the test setup for accelerated testing of various AA-size primary batteries to have a rough idea about their performance vis a vis their price. Our testing was simple. Different batteries were put on the same load (2.5 ohms). The voltage of each battery was continuously fetched by a microcontroller (PIC16F877A). The microcontroller sent the real-time voltage levels of all the battery cells to a MATLAB program running on a computer via serial port. The program saved these values in an Excel sheet for all the cells. The program stopped the process when all the batteries’ voltage fell below the minimum predefined level. The program provided the result in number of hours it took to discharge up to that level. Here 0.8V was taken as the minimum predefined value.
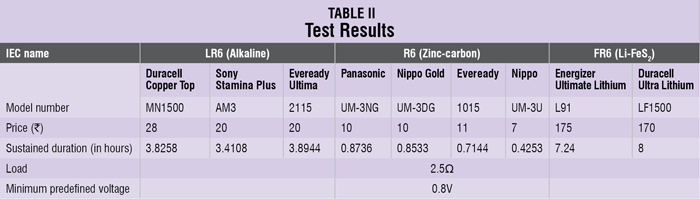
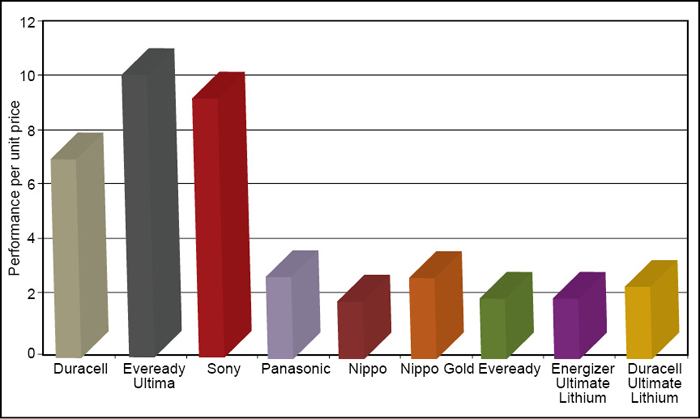
Table II shows the test results for different makes and chemistries. The data shows that Eveready Ultima (LR6) gave the best performance per unit price as shown in Fig. 3.
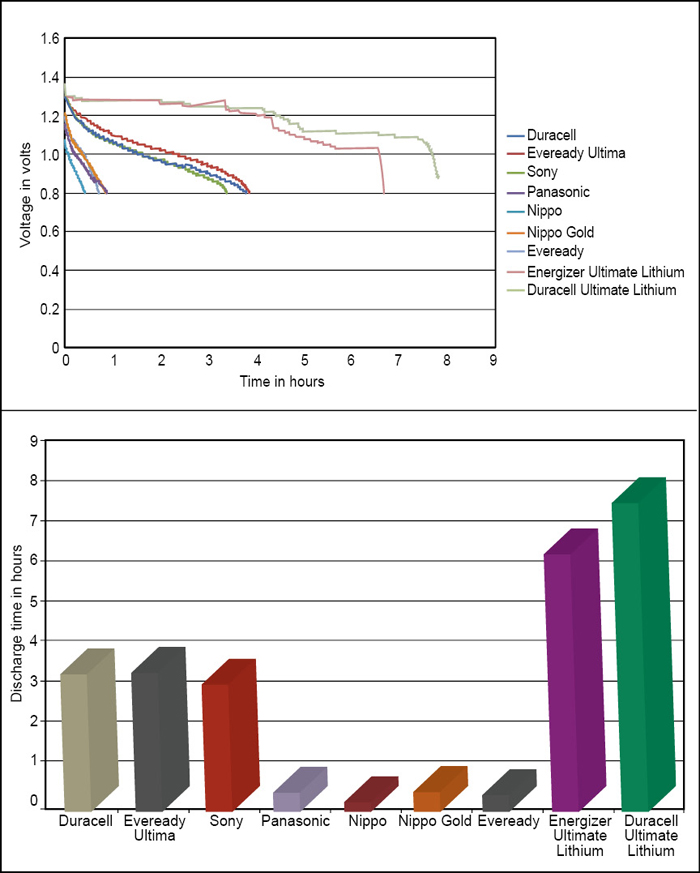
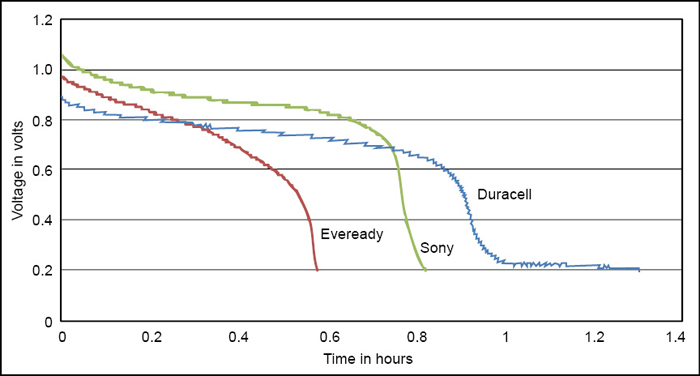
Fig. 4 shows the real-time graphs generated by the software. But when the test was run further in the range of 0.8V to 0.2V, the Duracell sustained much longer amongst all alkaline (LR6) batteries. Other batteries degraded very quickly after 0.8V but Duracell sustained much longer as shown in Fig. 5.
The author is a B.Tech in electronics and communications from SRCEM College, Gwalior






