Designing electronic medical devices requires engineers to walk an extra mile. After all, it’s the matter of saving lives! Since accuracy and reliability are crucial for medical equipment, designers have to follow a strict set of regulations for their designs. While this norm has been a constant in this vertical, there have been many changes and upgradations in terms of form factor, usage and technology incorporated in devices. This article reviews all these upgrades and essentials in medical electronics design.
New technologies
Medical devices—both diagnostic and treatment types—have seen a shift in design preferences over the years. The combination of electronics, mechanical engineering, medical science and software engineering governs the progress of these equipment.
Nitesh K. Jangir, co-founder, Coeo Labs, a Bengaluru-based medical equipment manufacturer, says, “Design starts from the hardware. Most of the medical devices use embedded software. So hardware requires more attention. You need to know what kind of sensors, components and MCUs you will be using.”
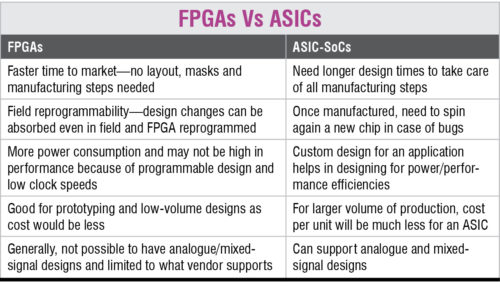
For medical devices, application-specific integrated circuit (ASIC) system-on-chips are preferable over field-programmable gate array (FPGA) chips—the reason being that although FPGA offers more customisation options through simple designs, ASICs provide higher power efficiency, better performance and purpose-specificity. Upgraded image processing technologies have improved different applications such as computed tomography and magnetic resonance imaging scan tests.
Miniaturisation and portability is another major trend in medical devices. To ensure easy handling as well as quick diagnosis, many critical equipment like ultrasound machines and electrocardiograms (ECG) are being designed in portable shape. This has made diagnosis of commute-affected patients much easier.
Even ventilators are becoming portable. One of the newest examples is a portable ventilator designed by professor Diwakar Vaish, head of robotics and research, A-SET Training and Research Institute, in collaboration with Dr Deepak Agarwal, professor of neurosurgery at AIIMS, New Delhi. Its main components include pressure sensor monitors and a special microcontroller unit that operates on a program based on machine learning algorithm.

As regards connectivity, Nitesh says, “Almost all medical devices are becoming connected to the Internet. This is giving users the freedom to utilise data from anywhere. For instance, users are adopting ECG devices with Bluetooth connectivity to access ECG data through their phones. We as designers try to ensure that every device at least has the option to connect to the Internet. Wi-Fi, GSM and Bluetooth are the main wireless technologies being utilised. So we integrate the respective modules with the circuit board.”
“Globally, Zigbee is widely used in medical equipment. In India, there are a handful of companies using Zigbee. These companies use Zigbee for medical equipment mainly because their Wi-Fi is occupied for other applications. Once again, keeping this in mind, medical devices are being designed with multiple wireless platform connectivity modes,” he adds.

Essential design tips
Medical device designs need to meet several regulations and pass safety compliance tests. Often, designers face the challenge of rework after putting up a device that misses some safety regulations.
Nitesh explains, “All components (including regulators and capacitors) should be certified or approved by regulatory agencies like ISO and IEC. This may add to the cost, but it is a necessity. If your design uses radio-components, these should also have a regulatory certificate.”
Appropriate hardware component selection is crucial. While sensors vary from device to device depending on the intended application, microcontroller remains the brain of computation. Choosing the right microcontroller is imperative to designing a device appropriate for its purpose.
“In life-critical devices, processors with backup cores should be preferred. In case of a failure in the main core, the alternate core will keep the device running. For example, ARM Cortex R-series are becoming the microcontrollers of choice. That’s because these have an in-built safety mechanism that gets activated in case of any failure,” says Nitesh.
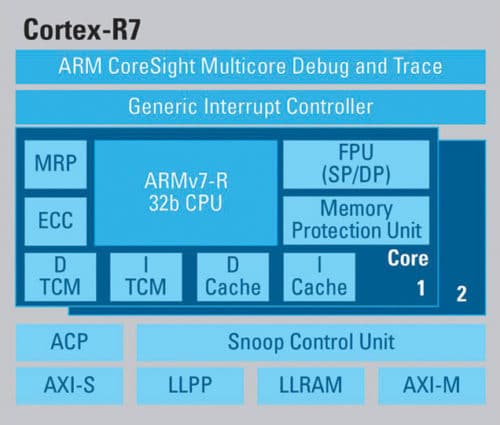
Nitesh adds that companies like Texas Instruments are building MCUs exclusively for medical devices. Many medical devices require low-amplitude data signals, say, 24-bit, and that is the kind of design these controllers follow.
Another important design aspect is safety. Electrical insulation should also be very thorough.
Jangir says, “You should know the amount of insulation required for your hardware. For instance, for a specific test, the device should stay stable at 3kV line voltage. Moreover, if you are designing a high-speed device, you should know how to properly ground the overall circuit. This will keep any kind of radiated emission (RE) as low as possible. Additionally, the hardware needs to be robust enough for any situation.”
The devices should be made electromagnetic-interference (EMI)-proof as much as possible. Electromagnetic compliance (EMC) can be ensured through electrical safety, mechanical safety and EMI safety.
Like any other electronic equipment, medical devices are prone to overheating. To make the device heat-resistant, first ensure proper grounding. Next, choose an appropriate heat-sink. Additionally, ensure sufficient ventilation in the design schematic. In the least likely scenario, you will need to introduce a fan into the device.
Test and measurement tools can ensure precision of medical data. For a customised medical device design, get your device tested and certified. Software for medical devices are mostly embedded. There are predefined regulations that guide developers to build software optimal for data safety and proper functioning.
To sum up
Compliance with defined regulations and selection of appropriate hardware components are crucial for optimal circuit design. With the inclusion of more powerful hardware, better processing units with safety protocols and wireless connectivity, the ever-evolving medical equipment keep getting safer and more reliable. It would be interesting to see how these further incorporate even newer and more intelligent technologies.