When scarcity of lithium required for lithium-ion batteries is affecting large-scale production of these batteries, the emergence of alternatives such as sodium-ion batteries is gaining importance. But will these be able to replace the lithium-ion batteries soon enough and prove better in the long run?
In the battery dependent lifestyle that is the norm today, the coming of lithium-ion batteries was considered as a great breakthrough. Offering many advantages related to capacity, compactness, and life cycle, there is no doubt that lithium-ion-battery-chemistry reigns supreme in manifold electronic devices. However, of late, practical experiences with these batteries along with growing concerns over cost, safety (towards fires and explosions), and lithium production limitations are casting a doubt on the future of these batteries.
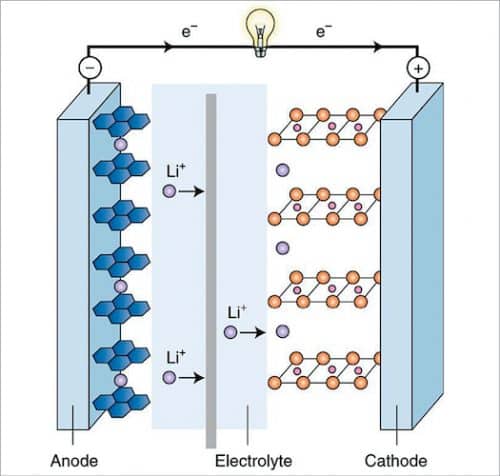
Scientists, engineers, and technologists are constantly thinking about other advantageous battery options, and one possible alternative for lithium-ion (Li-ion) batteries is sodium-ion batteries. Recently, sodium-ion (Na-ion) batteries have emerged as another viable means of energy storage, mainly for large-scale electric storage applications. This is primarily due to low cost and easy availability of sodium as compared to lithium, while having similar chemistry and intercalation kinetics to that of lithium battery and the irreversible capacity of carbon anodes.
Recent research developments like those to increase sodium storage behaviour via electron-rich element-doped amorphous carbon are aiming to make Na-ion batteries a more affordable and sustainable replacement to Li-ion batteries. Therefore, Na-ion batteries are a recent development being promoted repeatedly not only as an economically promising alternative to lithium-ion batteries but also as one with high-performance and safe battery options.
Comparison with lithium-ion batteries
Conventional Li-ion batteries with liquid electrolyte can store high amounts of energy, whereas solid-state Na-ion batteries with a solid electrolyte core is unable to produce the same amounts of energy. Recent research however, has brought out a solid electrolyte that is as conductive as the liquid electrolytes used in lithium-ion batteries.
The last challenge towards achieving a highly efficient solid-state Na-ion battery was to find solid interfaces. Addressing this, the research has two main findings. The first is that the resistive interface between the electrolyte and the cathode commonly formed during the cycle can be reversed, thus extending the cycle life. The second is related to the flexibility of the organic cathode which allows it to maintain intimate contact at the interface with the solid electrolyte, even as the cathode expands and contracts during the cycle. This serves as a solution to one of the major problems till now.
The organic cathode is much more flexible, maintaining contact at all times with the interface and improving cycle life. According to the researchers, the contact between the electrode and electrolyte remained steady through at least 200 cycles, and this reliable contact will have a great chance of creating a high-performance solid-state battery.
Will sodium replace lithium?
Though Li-ion batteries play an important role in the transition towards a low-carbon economy, as the market rapidly expands, so does the need. The environmental and social challenges associated with the mass production of Li-ion batteries is triggering attention towards the search for alternative energy storage solutions based on materials that can be sourced in a sustainable and responsible way.
Na-ion batteries have great potential to represent the next-generation low-cost and environmentally friendly energy storage solution. The varied key performance indicators required by different applications and the market diversification are the driving forces pushing the Na-ion technology closer to the market. Na-ion batteries offer a combination of attractive properties as they are low-cost, use sustainable precursors, and have secure raw material supplies.
Na-ion batteries do not represent a massive departure from their Li-ion variants as the elemental structure of sodium is quite similar to lithium (being a Group 1 metal); thus material testing processes are similar and manufacturing processes are comparable too. Sodium is many magnitudes more abundant than lithium and is found everywhere in the Earth’s crust and is harvestable from the ocean. Thus, Na-ion batteries are considered as a drop-in technology that could benefit from the already existing Li-ion batteries manufacturing facilities.
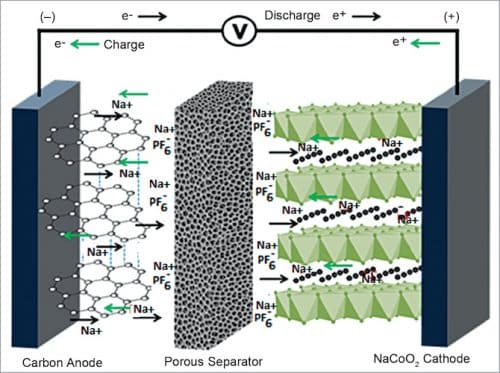
Similar to Li-based systems, Na-based batteries also come in different forms such as Na-ion, Na-all-solid-state-battery, NaO2 and Na/S. Na-ion technology represents an attractive technology almost ready to challenge the Li-ion batteries in specific applications, while the other ones are seen as disruptive future technologies.
A promising solution for stationary energy storage systems
According to researchers working for the development of batteries, with the ongoing developmental growth, the present best materials available for Na-ion cells should allow approaching the energy density of the present generation of Li-ion commercial cells. At present, sodium batteries are not quite viable for personal, portable electronics but are better suited for stationary applications.
One of the most significant application areas for the Na-ion battery prototype is certainly stationary energy storage systems, where cost and cycle-life represent two fundamental parameters. They have the potential to dominate the future market by representing as the most promising system to fill the gap between energy production and utilisation by securing energy supply.
At lesser cost, consuming lesser resources
Ironically, the similarities between both chemistries and production strategies are a boon for Na-ion batteries. While cobalt isn’t as integral to Na-ion batteries, sodium cobalt oxide (NaCoO2) remains a common cathode material and researchers have been able to develop a new sodium oxide cathode material.
Additionally, these experimental sodium-ion batteries retain capacity, resist moisture, and have little voltage-fade, which is a common phenomenon with prolonged lithium battery cycles. Thus, Na-ion rechargeable batteries could soon be a cheaper and resource-saving alternative to current lithium-ion batteries.
As the research stands
At present, the electromobility version of transportation, especially in combination with renewable electricity utilisation, is regarded world-wide as a climate-friendly model of transportation for the future. However, the intense focus on the use of the rare and expensive lithium metal in rechargeable batteries has slowed down the large-scale electrification of vehicles across the world. It is well known that for electric vehicles to come into more widespread use as per need, the future batteries must become even more powerful, durable, sustainable- and cost-effective.
The solution, in this regard, could be the emergence of Na-ion batteries, whose development has recently made astonishing progress. It is foreseen that future Na-ion batteries could replace the Li-ion batteries not only in electric vehicles but also in smartphones and laptops. Although the two alkali metals lithium and sodium are chemically very similar, sodium does not have the energy density of the comparatively rare lithium, but it is widely and cheaply available. Research activities on Na-ion battery materials have increased significantly in the past decade, with the key motive to find a reliable, safe- and cost-effective substitute for Li-ion batteries.
However, there are some key differences in the electrochemical behaviour of both battery systems. For example, the cell voltage of sodium half-cell is lower than that of lithium by 0.3V, while Na-ion conductivity is higher than Li-ion due to its polarisability. Overall, it is encouraging and helpful that most of the research carried out on Li-ion based systems has been successfully transferred to Na-ion batteries to explain and improve the fundamentals of various physio-chemical and electro-chemical phenomena within the battery system.
The verdict: Na-ion batteries have the potential to compete with Li-ion batteries
Based on different performance parameters, Na-ion batteries have the potential to compete with Li-ion batteries in three main areas of applications, starting from small to medium and large scale applications. Applications involving portable electronic devices which are presently powered by Li-ion batteries require a capacity in the range of 200-300mAhg–1, with cyclic life of over 200 cycles and capacitive retention of more than 80%.
From available research data and expected improvements in electrode/electrolyte materials, with the added advantage of lower raw material cost of Na-ion batteries, it would be safe to say that these will be strong competitors to Li-ion batteries in the near future. For a long time, research has been concentrated solely on the more powerful Li-ion batteries. Now, however, there are not only ground-breaking scientific publications, but also very promising prototypes coming of sodium-ion batteries. Some examples:
- A South Korean developed Na-ion battery has managed to handle about 500 complete charging cycles before its capacity dropped to 80%.
- A battery with a slightly different chemical structure designed by a US-Chinese research group has achieved 450 charge cycles with a similar charging capacity.
- A Chinese-prepared sodium-ion battery had a slightly lower capacity but still retained 70% of its capacity after 1,200 cycles of quick 12-minute charging.
Most sodium-ion batteries still use graphite as the anode material, making them less proficient. However, Korean researchers have now crafted new designs using modified carbon anodes which have yielded thermodynamic benefits and boosted capacity. This move promises to offset the technology’s shortcomings and scientists sought to do the following:
- Promote rapid electron transport from electrolyte to active material via a porous separator.
- Make sodium ions available in large numbers to the active material across larger sites.
- Enable structured co-intercalation (ion insertion) from surface to interior.
- Maintain short diffusion paths and micro-structures.
- Increase the number of active sites.
A sodium-oxide cathode alternative to cobalt
With the current level of performance, Na-ion batteries lag behind Li-ion batteries by about twenty years, but in practice, these batteries would probably survive many more charge cycles. Moreover, the production of the cathodes does not require rare lithium salts as simple table salt is sufficient to make a battery. Powerful anodes can also be produced from lignite, wood, and other biomass, making cobalt or similar rare substances unnecessary.
Challenges to be overcome
The chemistry and electro-chemistry of electrode materials for sodium-ion batteries are sufficiently different from that of their lithium counterparts, making them suitable candidates for practical batteries. The limited electrochemical stability window of water results in lower voltages and limited energy densities when aqueous electrolytes are used in Na-ion batteries.
Moreover, the disadvantages of sodium are that it is three times heavier than lithium, making Na-ion batteries also heavier. Further, sodium batteries are less powerful because they inevitably lose around ten percent of their energy density due to a 0.3V lower cell voltage, largely owing to the fact that the graphite anodes used up to now in batteries absorb too little sodium.
Recent studies by a German-Russian working group have shown that nanoscale carbon could provide a remedy to this problem. The study suggested that double layers of graphene, which is wafer-thin carbon, could store significantly more sodium atoms in the anode than the graphite used so far for this purpose.
Further technological improvements are needed to increase the performance, especially in terms of energy density as well as the technological improvement in cell-component fabrication and assembly optimisation. The future research has to be planned towards fundamental research, materials discovery, and understanding of the thermodynamic and kinetic processes governing the chemistry of these systems. In addition, the investigation to scale up Na-ion batteries is of primary importance to obtain realistic data to benchmark the progress of the technology.






