Lithium battery packs are particularly sensitive to faults caused by external shorts, runaway charging conditions and abusive overcharging, which can cause potentially damaging over-current and over-temperature conditions. Hence, there is a need to enforce safety regulations and established test requirements for lithium-ion and lithium-polymer packs to demonstrate their resilience to both short-circuit and overcharge events.
Nickel-cadmium had been the only suitable battery for the last many years, to be used in portable equipment from wireless communications to mobile computing. Then, other types of batteries like nickel-metal-hydride and lithium-ion emerged in the early 1990s, giving fierce competition to gain customer’s acceptance. Evolution of battery technologies is directly tied to miniaturisation of mobile electronics, and to power portable electronic devices in everyday life. Lithium-ion rechargeable batteries are extremely popular and can be found in laptops, PDAs, cellphones and iPods.
Today, lithium-ion has emerged as the fastest growing and most promising battery chemistry. Lithium-ion battery technology is further driven by consumer demands for applications that have more computing power, and come in thinner and smaller packages. In response, design engineers and components manufacturers must continue to find creative solutions that conform to these requirements.
Groundwork towards the development of lithium-ion battery started during the oil crisis of 1970s. Prof. M. Stanley Whittingham (USA) was working towards developing methods that could lead to fossil fuel-free energy technologies supported with research on superconductors. He discovered an extremely energy rich material from titanium-disulphide to create an innovative cathode in a lithium battery. This material, at molecular level, has spaces that can store intercalated lithium ions.
Anode of the battery was made partially from metallic-lithium having strong drive to release electrons. With present batteries offering potential much less than a volt even, such a combination of anode and cathode materials resulted in a battery with a great potential (just over two volts). However, the developed metallic lithium battery was reactive and too explosive to be viable.
Contemporary to this research, Prof. John B. Goodenough (USA) predicted that cathode made up of a metal-oxide instead of a metal-sulphide would have even greater potential. After a long systematic research, Prof. Goodenough in 1980 demonstrated that cobalt-oxide with intercalated lithium ions can produce potential as much as four volts. This was an important breakthrough in the history, leading to development of much more powerful batteries.
Making the research on cathode by Prof. Goodenough as the basis, Prof. Akira Yoshino (Japan) created the first commercially viable lithium-ion battery in 1985. Instead of using reactive lithium in the anode, he made use of petroleum coke (a carbon material) that like cathode of cobalt-oxide can intercalate lithium ions. Use of petroleum coke cathode resulted in a lightweight and hardwearing battery that could be charged hundreds of times before its performance deteriorated, thus leading to development of rechargeable batteries.
Lithium-ion batteries are not based upon chemical reactions, leading to the breakdown of electrodes; rather, these work on the basis of lithium ions flowing back and forth between anode and cathode. Since entry of lithium-ion batteries to the market in 1991, these have revolutionised every aspect of our lives based on the use of batteries and have laid down the foundation of a wireless, fossil fuel-free society.
The Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry 2019 to Profs John B. Goodenough (USA), M. Stanley Whittingham (USA) and Akira Yoshino (Japan) for the development of lithium-ion batteries. These lightweight, rechargeable and powerful batteries are now used in everything from cellphones to laptops and electric vehicles (EVs). These types of batteries can also store significant amounts of energy from solar and wind power generators, thus leading to a fossil fuel-free society.
Electronics with lithium-ion battery
Lithium-ion battery, having much better energy density (joules of energy per kilogram) than previous battery technologies like nickel-cadmium (NiCd) and nickel-metal-hydride (NiMH), makes use of portable electronics more versatile to mankind. Lithium is not the most stable element, so many new safety concerns arose with the introduction of lithium-ion batteries.
Sophisticated protection electronic circuits have been designed and developed to make lithium-ion batteries safe to use. Lithium-ion 18650 cells, which are shaped like cylinders (with 18mm diametre and 65mm length), are commonly used in present-day laptops, whereas typical notebook computers need battery packs comprising an average of about four to six 18650 cells to allow it to operate for several hours between charges.
Then about a decade ago, lithium-polymer (lipo) cells made their entry to the consumer market. Because of their attractive size characteristics like thin, rectangular, customised pouch-like shape, these packs were very attractive to designers looking for space savings, especially in ultra-slim electronic devices. Presently, lithium-polymer cells are being used in thin and large-screen electronic devices like smartphones and slimmer notebooks combined with high-end processors to run complex programs while still providing instant-on capability.
All of these trends in consumer electronics have driven the demand for larger, higher-capacity lithium-polymer cells with higher discharge currents. These demands and applications are fulfilled with higher current ratings in battery protection devices.
Working of a lithium-ion battery
Lithium is not only the lightest of all metals but also has the greatest electrochemical potential and, thus, provides the largest energy density by weight. Because of the inherent metallic instability of lithium, initial efforts to develop rechargeable lithium batteries failed miserably due to safety reasons.
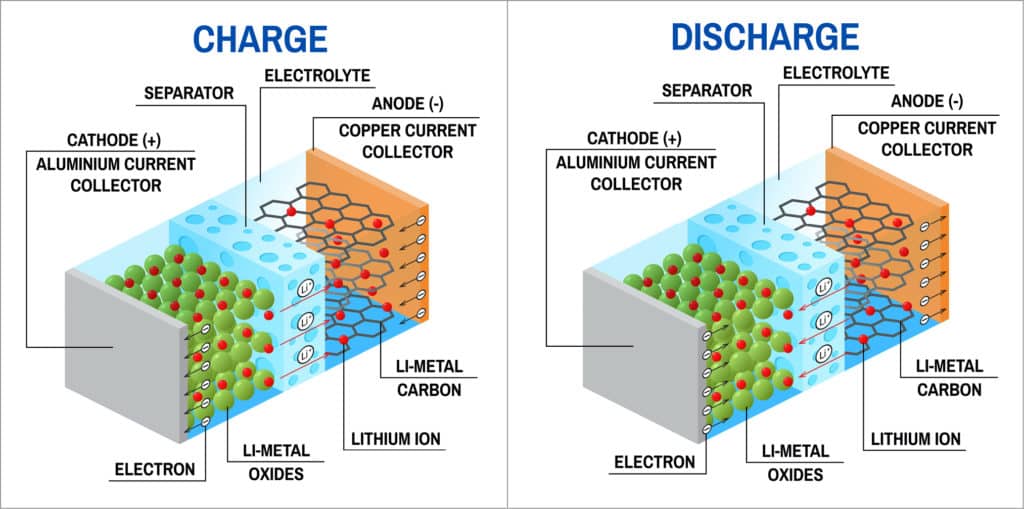
Research then shifted towards a non-metallic lithium battery using lithium ions. Lithium-ion battery is slightly lower in energy density than lithium metal but is safe taking into consideration certain precautions while charging and discharging of the battery. As with most batteries, lithium-ion battery also has an outer case made of metal, which is particularly important here because the battery is pressurised. Metal case of the lithium-ion battery, thus, has some kind of pressure-sensitive vent hole and, if the battery ever gets so hot that it risks exploding from over-pressure, this vent releases the extra pressure acting as a safety measure. The vent acts as a positive temperature coefficient (PTC) switch keeping the battery safe from overheating. The metal case holds a long spiral comprising three thin sheets pressed together as positive electrode, negative electrode and separator, which are submerged in an organic solvent acting as the electrolyte.
Ether is one common solvent used in lithium-ion batteries. Positive and negative electrodes are separated by a very thin sheet of micro-perforated plastic, allowing ions to pass through. The positive electrode is made of lithium-cobalt-oxide (LiCoO2), while the negative electrode is made of carbon. During the battery charging process, ions of lithium move through the electrolyte from the positive electrode to the negative electrode and attach to the carbon. Whereas, during discharging, lithium-ions move back to LiCoO2 from carbon. Movement of lithium ions takes place at a fairly high voltage making the cells produce 3.7 volts, which is much higher than the 1.5 volts typical of a normal AA alkaline cell.
Lithium-ion charge/discharge basics
Charging and discharging characteristics of lithium-ion battery are important, especially for its safe operation and long-term performance. To manage smooth and safe charging and discharging processes of lithium-ion battery, a typical battery management chip is incorporated into the battery pack. This way the user can plug the battery into a charger and leave it to charge with the knowledge that it does not have to be unplugged after a certain time. Moreover, the battery management chip also ensures that the battery is not discharged beyond limits so that it can be recharged easily. The focus is to ensure that the battery management pack understands the exact state of battery charge.
Charging lithium-ion batteries is different from charging Ni-Cads or NiMH batteries. Charging of lithium-ion batteries is voltage-sensitive rather than current-sensitive. Charging of lithium-ion batteries is same as charging of lead-acid batteries, only difference being lithium-ion batteries have a higher voltage per cell. These also require much tighter voltage tolerance on detecting full charge, and once fully charged these do not allow or require to be trickle or float charged.
Consumer-oriented lithium-ion batteries mostly charge to a voltage of 4.2 volts per cell with a tolerance of around ±50mV per cell. Charging of batteries beyond this voltage causes stress to the cell, resulting in oxidation, which reduces service life and capacity of the battery. Therefore it is important to be able to detect the full charge state of battery accurately because lithium-ion batteries do not tolerate overcharging, which can also cause safety issues.
For lithium-ion batteries, the amount of charge retained by the battery or cell against the amount of charge entering the cell (known as charge efficiency) is high, and charge efficiencies of around 95 to 99 per cent can be achieved with low-level temperature rise of cells. Charging process of lithium-ion batteries can be separated into two main stages, as given below.
Constant current charge
In the first stage of charging a lithium-ion battery or cell, charge current is controlled, which typically is between 0.5C and 1.0C. Capacity of the battery is commonly rated at 1C, meaning that a fully charged battery rated at 1Ah should provide 1A for one hour, and for a 2000mAh battery, charge rate would be 2000mA for a charge rate of 1C.
For consumer-based lithium-cobalt (LCO) cells and batteries, charge rate of a maximum of 0.8C is recommended. Thus, during charging process, voltage across the lithium-ion cell increases for constant current charge, and charge time may be around an hour.
Saturation charge
After some time, voltage peaks at 4.2 volts for an LCO cell, and at this stage the cell or battery must enter a second level of charging, known as saturation charge. Constant voltage of 4.2 volts is maintained and current steadily falls. End of the charge cycle is reached when current falls to around ten per cent of rated current. In this case, charge time may be around two hours depending on cell type.
Lithium-ion batteries’ charging/discharging characteristics show improvements over its competitors, and have been shown to be able to withstand over one thousand charge/discharge cycles but still be able to hold eighty per cent of initial capacity.
NiCd batteries offer up to around 500 cycles, which is very much dependent on the way batteries are used, and a badly treated cell may only give fifty or hundred.
NiMH cells are even worse, and this is one of the main areas receiving development. These are only able to give 500 cycles at the very best before their capacity drops to eighty per cent of initial charge rating.
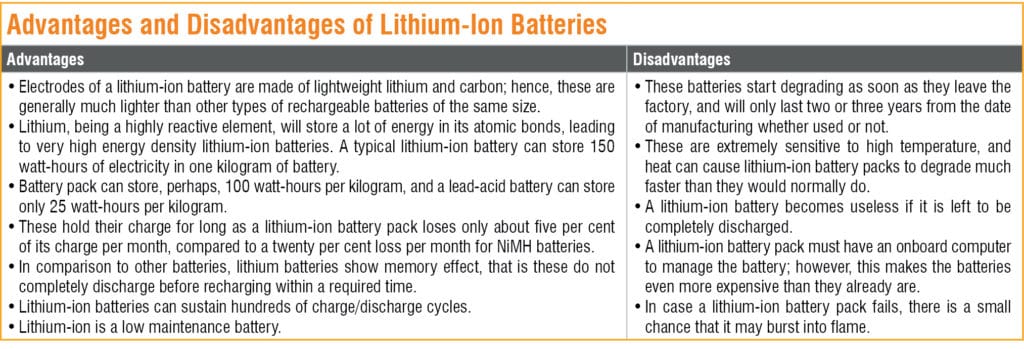
Due to a number of significant advantages associated with lithium-ion batteries in comparison to competing technologies, these batteries are becoming popular but are not without limitations. The advantages and disadvantages of lithium-ion batteries are summarised in Table 1.
Lithium-ion battery charging precautions
In view of the amount of energy stored in lithium-ion batteries and the nature of their chemistry, etc, it is necessary to ensure that the batteries are charged in appropriate manner and with an appropriate charger and equipment. Battery chargers or battery packs have inbuilt mechanisms to prevent damage and dangers due to overheating. Mechanisms required for charging and discharging of lithium-ion batteries include:
Charge current
Charge current must be limited for lithium-ion batteries with a typical maximum value of 0.8C, but lower values are usually set to give some margin.
Charge temperature
Lithium-ion battery charge temperature should be monitored, and cell or battery must not be charged when temperature is lower than 0°C or greater than 45°C.
Discharge current
Discharge current protection is required to prevent damage or explosion as a result of short-circuits.
Over-voltage
Charge over-voltage protection is required to prevent voltage that is too high to be applied across battery terminals.
Over-charge protection
Over-charge protection circuitry is required to stop the lithium-ion charging process when voltage per cell rises above 4.3 volts.
Reverse polarity protection
Lithium-ion battery reverse polarity protection is needed to make sure the battery is not charged in the wrong direction as this could lead to serious damage or even explosion.
Lithium-ion over-discharge
Over-discharge protection is required to prevent battery voltage falling below about 2.3 volts or dependent on manufacturing of the cell.
Over-temperature
Over temperature protection is often incorporated to prevent the battery from operating if temperature rises too high, and temperatures above 100°C can cause irreparable damage to the battery.
Battery protection challenges
If the battery gets hot enough to ignite the electrolyte, a fire is usually caused by an internal short in the battery. Lithium-ion cells contain a separator sheet that keeps positive and negative electrodes apart. If that sheet gets punctured and electrodes touch, the battery heats up very quickly. Heat produced can cause the battery to vent the organic electrolyte or light it. In a separator failure, some kind of short happens inside the lithium-ion battery. Once that happens inside one of the cells, heat of the fire cascades to other cells, and the whole pack goes up in flames.
Lithium battery packs are particularly sensitive to faults caused by external shorts, runaway charging conditions and abusive overcharging, which can cause potentially damaging over-current and over-temperature conditions. Hence, there is a need to enforce safety regulations and established test requirements for lithium-ion and lithium-polymer packs to demonstrate their resilience to both short-circuit and overcharge events.
Protection circuits built into the battery pack limit the peak voltage of each cell during charging as well as prevent cell voltage from dropping too low on discharging. In addition, cell temperature is also monitored with the help of protection circuits acting as temperature sensors to prevent temperature extremes as maximum charge and discharge current on most pacts is limited to between 1C and 2C. Using such precautions, the possibility of metallic-lithium plating due to overcharging is virtually eliminated. A few battery protection methods are summarised below.
- Temperature sensors to monitor battery temperature.
- Voltage converter and regulator circuit to maintain safe levels of voltage and current.
- Shielded notebook connector that lets power and information flow in and out of battery pack.
- Voltage tap to monitor energy capacity of individual cells in battery pack.
- Battery charge state monitor to handle the whole charging process to make sure battery charges as quickly and fully as possible.
- Battery storage in a cool place that slows the aging process of lithium-ion (and other chemistries).
Future scope
Twenty-five years ago, the lithium-ion battery made its debut into the marketplace as a result of innovative work by Asahi Kasei, and development and marketing by Sony Corp. Since the realisation of lithium-ion batteries, there has been tremendous development in terms of their capacity, energy, power and cost reduction.
Lithium-ion batteries have become indispensable today when we talk about the life quality of people in modern society. It can easily be called a dominant technology that is widely used in portable electronic devices. Lithium-ion batteries are also a preferred option today in the emerging sector of EVs. The technology is still on the breakthrough for its use in the field of power supply systems like wind power and photovoltaics. Thus, the technology offers a massive global potential in terms of reduction in carbon emissions and energy sustainability. Although these batteries show a lot of promise to rid the world of several challenges in the future, there are still a few shortcomings of this battery that need to be addressed.
Safety of lithium-ion batteries remains an important concern for the industry. However, new developments in separator technology have improved the outlook towards safer batteries. Similarly, with recent progress in new materials and successful implementation of new battery concepts in active materials, inert materials and cell designs, the lithium-ion battery will continue to improve in all its properties.
Researchers and manufacturers are constantly improving on lithium-ion batteries, introducing new and enhanced chemical combinations every six months or so. With such rapid progress, it will be possible to develop new lithium-ion batteries free from existing disadvantages.
Finally, several investigators are studying the possibility of 3D architecture of lithium-ion battery structures including porous or expanded metal collectors. This would help to increase battery density and spatial utilisation if production-friendly concepts are developed. The result of these advances, if realised, could yield lithium-ion battery with specific energies of 400Wh/kg with at least moderate power density. This would represent an increase of about sixty per cent compared to the best of today’s 18650 cells with 3400mAh capacity, and could come about within the next several years. Hence, the future for lithium-ion battery continues to be bright, especially if manufacturers are careful to maintain safe practices in manufacturing processes and new designs.
Dr S.S. Verma is professor at Department of Physics, Sant Longowal Institute of Engineering and Technology, Sangrur, Punjab












