Batteries are the dominant technology for powering portable devices. But the problem with batteries is their size and requirement of frequent charging, time required for charging, and repair and replacement.
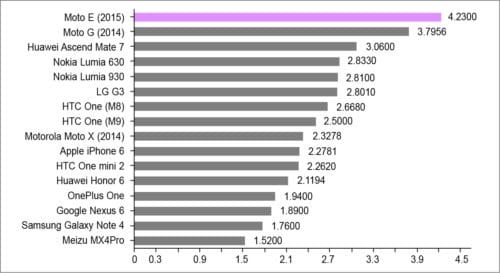
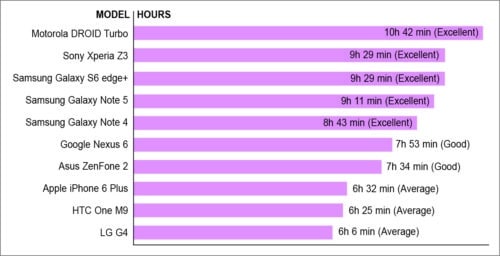
Fig. 1 shows the charging time for the batteries used in some mobile phones. These batteries discharge within hours of use as shown in Fig. 2. Capacitors are also energy storage elements that, unlike batteries, generate an electrical field between two parallel conductor plates. The energy stored in a capacitor depends on the value of its capacitance and the voltage applied across its plates. For the same voltage the capacitor will store more energy for a large value of capacitance. Since the capacitance of a capacitor is directly proportional to surface area of its electrodes and inversely proportional to the distance between electrodes, it requires a large area to store charge.
Fig. 1: Charging time of different batteries used in mobile phones
The current trend in portable devices is continuous miniaturisation, while ensuring the functionality and reliability of existing components. The main problem faced in miniaturisation is the integration of batteries as energy storage elements. The size of energy storage elements often limits the miniaturisation of the entire system. This is because the necessary energy-storage components cannot be scaled down to the size of electronic gadgets. So designing efficient miniaturised energy storage devices for energy delivery or harvesting with high-power capabilities remains a challenge.
Fig. 2: Battery life of mobile phones
Supercapacitors or ultracapacitors
An improvement in energy storage devices has been the development of electric double-layer capacitor (EDLC), best known as ‘supercapacitor’ or ‘ultracapacitor.’ A supercapacitor has large-surface-area conductive plates (electrodes), placed very close to each other.
Supercapacitors provide many advantages over batteries. For instance, these have a long cycle lifetime—they can be cycled hundreds of thousands times without much change in performance. The lifespan of a supercapacitor is 10 to 20 years, with a possible capacity reduction from the original 100 per cent to 80 per cent after ten years of usage or so.
Supercapacitors have a low equivalent series resistance. These can therefore provide a high power density and high load currents to achieve almost instant charge in seconds.
The new hybrid supercapacitors store large amounts of energy, recharge quickly and last more than 10,000 recharge cycles. This is a great advantage for smartphones, which will charge in just 30 seconds and then continue working through the rest of the day on a single charge.
Basic principle of operation
The basic principle of energy storage in supercapacitors is the same as that of ordinary capacitors. However, unlike conventional capacitors that use a solid and dry dielectric material, such as teflon, polyethylene and paper, ultracapacitors use a liquid or wet electrolyte between their electrodes. A supercapacitor or electric double-layer capacitor, as shown in Fig. 3, utilises large-surface area electrodes and thin electrolytic dielectrics to achieve capacitance values greater than any other capacitor type available today.
Energy storage in capacitors is by means of static charge and does not involve the electro-chemical process inherent to batteries. These store charge as a coating of ions adsorbed on the electrodes’ surface. Ions are separated from the electrolyte by a charging current, and propelled toward their respective electrodes. The membrane serves to separate the ions, so that a net charge separation can be maintained. This dual surface layer is called electric double layer.
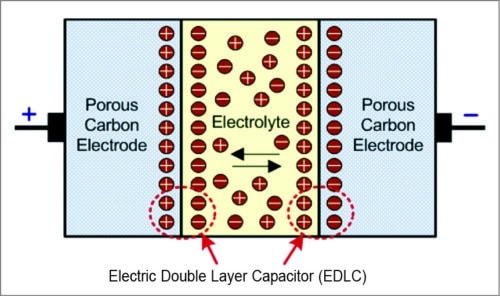
Fig. 3: Electric double-layer capacitor (supercapacitor)
Supercapacitors have separation of charge at an electric field which is only fractions of a nanometre, compared to micrometres for most polymer film capacitors. In doing so, supercapacitors are able to attain greater energy densities while still maintaining the characteristic of high power density of conventional capacitors. These are therefore more of an electrochemical device similar to an electrolytic capacitor. They store charge through reversible ion adsorption at the surface of high-surface-area carbon. Different materials, such as various carbon materials, mixed-metal oxides and conducting polymers, have been used for supercapacitor electrodes. Advances in carbon-based materials, namely, graphene, have increased their energy density to almost the level of batteries.
Graphene is important
Graphene is a thin layer of pure carbon, tightly packed and bonded together in a hexagonal honeycomb lattice. It is widely regarded as the most suitable material for supercapacitors as it is the thinnest compound known to man at one atom thickness, as well as the best known conductor. It also has amazing strength and light absorption characteristics, and is even considered eco-friendly and sustainable, as carbon is widespread in nature and is a part of the human body.
Graphene-based materials are considered as one of the most promising materials for the next-generation, flexible, thin-film supercapacitors due to their unique structure and properties: Their two-dimensional structure can provide a large surface area, which serves as an extensive transport platform for electrolytes. The high conductivity of graphene sheets enables a low diffusion resistance, leading to enhanced power and energy density.
The superior mechanical property allows graphene sheets to be easily assembled into free-standing films with robust mechanical stability. Among graphene-based 2D films, graphene papers have attracted much attention due to their tunable thickness.
Challenge with integration
But as devices shrink, integrating the storage element as close as possible to the electronic circuit (directly on a chip) is another challenge. Electrochemical capacitors with a high surface-to-volume ratio of the active material have high energy and power densities; this is further enhanced in micro-supercapacitors.
Porous activated, templated and carbide-derived carbons, multi- and single-walled carbon nanotubes, onion-like carbon (OLC) and multilayer graphene have been used as electrode materials in supercapacitors. Progress in micro-fabrication technology has enabled on-chip micro-supercapacitors in an interdigitated planar form in contrast to the conventional sandwich structure. The planar form is compatible with integrated circuits. Micro-supercapacitors, besides their miniaturised structure, have high power density, high rate capability and high frequency response, which are crucial for most applications.
Among all the options available, only onion-like carbon has produced micro-supercapacitors capable of ultra-high power handling with an R-C time constant of only 26ms.
Onion-like carbon
Although OLC has been synthesised by many different methods in the last 30 years, large-scale production (gram quantities) of OLC was first realised in 1994 using vacuum annealing of a nanodiamond precursor. Annealing in inert gases to transform nanodiamond (currently produced in large quantities) to OLC is also used by many researchers. This method has a potential for industrial applications, as the onion yield is close to 100 per cent and the manufacturing volume is only limited by the size of the furnace, and can be scaled accordingly. This material rarely has ideal spherical carbon onions, but can be produced in large quantities for practical applications.
Another synthesis technique using arc discharge between two graphite electrodes in water produces OLC of slightly different structure than from annealing of nanodiamond. A DC current of 30A and 17V applied between two graphite electrodes in water causes the carbon to evaporate at the location of the arc due to the extreme heat generated. The carbon vapour rapidly condenses into highly spherical OLC particles.
Hollow carbon onions have been produced with the help of metal nanoparticles. One practical method for the fabrication of hollow carbon onions uses nitric acid to dissolve nickel from the carbon-coated nickel nanoparticles. In this method, the decomposition of methane in the presence of Ni/Al catalyst particles produces carbon nano-onions as the primary product.
Zero-dimensional nanomaterials
Carbon-encapsulated metal (magnetic) nanoparticles represent a new class of zero-dimensional carbon-metal composite nanomaterials. These materials are very easily purified to the form of hollow onions by the subsequent nitric acid treatment.
There are several other processes to produce carbon onions. Synthesis of carbon onions via chemical vapour deposition (CVD) utilises an iron catalyst supported on sodium chloride to decompose acetylene gas at 400°C temperature. Today, catalytic chemical vapour deposition is considered as the only economically viable process for large-scale carbon nanotube production and their integration into various devices. In contrast to other methods of carbon onion synthesis, this CVD process yields much larger-diameter (50nm) particles.
Carbon ion implantation is another method to produce carbon onions, which allows the particle diameter to be tuned from 3nm up to 30nm by varying synthesis conditions such as temperature or implantation dose density. Thermolysis, in which a compound is decomposed by heat, has been shown to be a method for carbon onion synthesis. Using sodium azide (NaN3) and hexachlorobenzene (C6Cl6) as reagents, a redox reaction causes an abrupt increase in temperature and pressure, producing large-diameter carbon nanotubes.
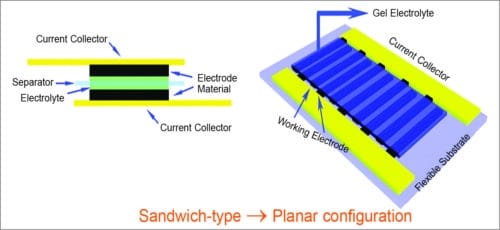
Fig. 4: Micro-supercapacitors
Recent progress in on-chip micro-supercapacitors
A novel method is being developed for fabricating micro-patterned interdigitated electrodes based on reduced graphene oxide (rGO) and carbon nanotube (CNT) composites for ultra-high-power-handling micro-supercapacitors applications.
There are two main approaches to fabrication of devices with interdigital electrodes. In the first approach, interdigital current collectors are fabricated and then the active materials deposited onto the current collectors using thin-film deposition method. In the second approach, a patterning step is performed on a thin film of active material to form the interdigital electrodes.