Researchers at Waseda University have developed a solid-state rechargeable air battery (SSAB) and conducted extensive investigations into its capacity and durability.
Metals are common in battery-negative electrodes, but redox-active organic molecules like quinone- and amine-based ones are now used in rechargeable metal-air batteries with oxygen-reducing positive electrodes. Protons and hydroxide ions engage in redox reactions, leading to high-performance batteries nearing the theoretical maximum capacity.
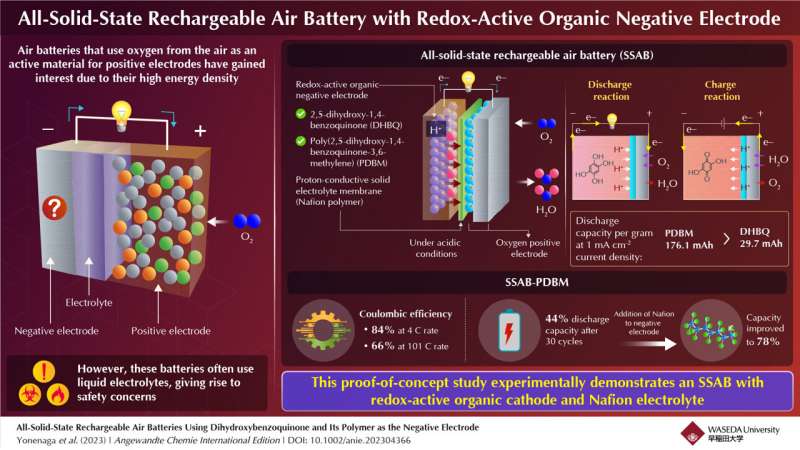
Japanese researchers at Waseda University have created a solid-state rechargeable air battery (SSAB) and studied its capacity and durability. Rechargeable air batteries with redox-active organic molecules offer advantages over metal-based batteries by avoiding issues like dendrite formation and negative environmental impact.
The researchers used 2,5-dihydroxy-1,4-benzoquinone (DHBQ) and its polymer poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene) (PDBM) as active materials for the negative electrode. These were chosen for their stable and reversible redox reactions in acidic conditions. They employed a proton-conductive polymer called Nafion as the solid electrolyte to replace liquid electrolytes. After implementing the SSAB, the researchers tested its charge-discharge performance, rate characteristics, and cyclability. Unlike conventional air batteries with metallic negative electrodes and organic liquid electrolytes, the SSAB exhibited no degradation when exposed to water and oxygen.
Substituting DHBQ with PDBM improved the negative electrode. SSAB-DHBQ had a discharge capacity of 29.7 mAh/g, while SSAB-PDBM reached 176.1 mAh/g at 1 mAcm-2 current density. The researchers observed that SSAB-PDBM had a coulombic efficiency of 84% at 4 C rate, which decreased to 66% at a 101°C rate. After 30 cycles, the discharge capacity of SSAB-PDBM decreased to 44%, but by increasing the proton-conductive polymer content in the negative electrode, researchers substantially improved it to 78%. Electron microscopic images confirmed that adding Nafion enhanced the performance and durability of the PDBM-based electrode.
The study shows the successful operation of SSAB with organic molecules as the negative electrode, a polymer as an electrolyte, and an oxygen-reducing positive electrode. The researchers aim to advance technology for longer gadget battery life and a carbon-free society.
Research: Makoto Yonenaga et al, All‐Solid‐State Rechargeable Air Batteries Using Dihydroxybenzoquinone and Its Polymer as the Negative Electrode, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202304366