Researchers at Flinders University in South Australia and Zhejiang Sci-Tech University in China have developed a safe, efficient, non-toxic aqueous aluminium radical battery.
Most batteries often contain harmful substances that threaten the environment when improperly discarded, such as in landfills or other disposal methods. Hazardous materials like lead, cadmium, and mercury can endanger humans and animals, contaminate soils and water sources, and persist in the environment for extended periods.
Researchers at Flinders University in South Australia and Zhejiang Sci-Tech University in China have developed a non-toxic aqueous aluminium radical battery, prioritising safety and efficiency.
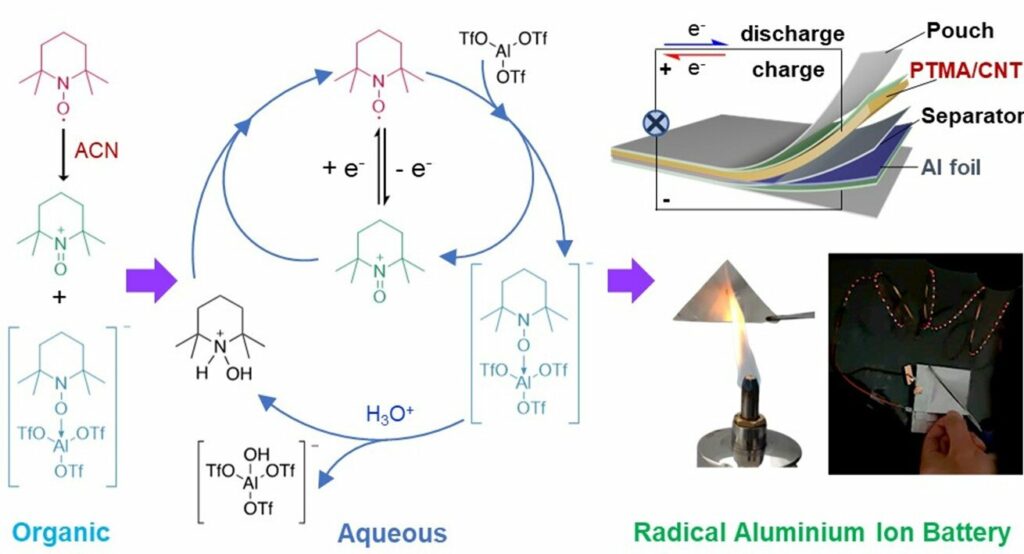
The team developed an initial design for radical aluminium batteries utilising water-based electrolytes that exhibit fire-retardant and air-stable properties. These batteries demonstrated a consistent voltage output of 1.25 V and a 110 mAh g–1 capacity throughout 800 cycles, with a mere 0.028% loss per cycle. The researchers envision using biodegradable materials in the future development of soft-pack batteries. This approach aims to enhance both the safety and sustainability of the product. According to the researchers, multivalent metal ion batteries, such as those employing Al3+, Zn2+, or Mg2+, utilise abundant elements in the Earth’s crust.
These batteries offer significantly higher energy density than lithium-ion batteries (LIBs).
Aluminium-ion batteries (AIBs) are a sustainable and affordable energy storage system due to the abundance of aluminium. However, one of the significant challenges for current AIBs is the slow movement of Al3+ ion complexes, resulting in low cathode efficiency. To tackle this ion transport problem, organic conjugated polymers have emerged as promising cathode materials for AIBs. However, their battery voltage output performance still needs to catch up. Stable radicals represent a category of organic electroactive molecules extensively employed in various organic battery systems.
The researchers have created radical materials for organic hybrid LIBs, sodium-ion batteries, and all-organic batteries. However, the application of these radical materials in AIBs has been limited due to a need for more understanding regarding their (electro)chemical reactions in electrolytes.
Reference: Shangxu Jiang et al, Lewis Acid-Induced Reversible Disproportionation of TEMPO Enables Aqueous Aluminum Radical Batteries, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c04203