This promises enhanced safety, extended lifespan, and superior performance at elevated temperatures and offers exciting prospects for high-temperature industrial applications and fast-charging electric vehicles, marking a significant step toward a sustainable energy future.

In recent years, batteries have become essential in consumers’ daily lives. Existing commercial battery technologies, which use liquid electrolytes and carbonaceous anodes, have certain drawbacks such as safety concerns, limited lifespan, and inadequate power density, particularly at high temperatures. There is an increasing demand for batteries that can operate in extreme conditions, such as the high temperatures required in various industrial sectors, including medical device sterilization, subsurface exploration, and thermal reactors. Researchers are therefore searching for solid electrolytes that are safe and compatible with lithium metal anodes, known for their high theoretical specific power capacity.
A research team led by Professor Dong-Myeong Shin of the Department of Mechanical Engineering at the University of Hong Kong (HKU) has made a significant advancement in this area. They have developed a new generation of lithium metal batteries featuring microcrack-free polymer electrolytes. These electrolytes promise extended lifespan and enhanced safety at elevated temperatures. The microcrack-free polymer electrolytes are synthesised via a straightforward one-step click reaction and exhibit notable attributes including resistance to dendrite growth and non-flammability. They demonstrate a high electrochemical stability window up to 5 V and an ionic conductivity of 3.1 × 10−5 S cm−1 at high temperatures. These enhancements are attributed to tethered borate anions within the microcrack-free membranes, which facilitate accelerated selective transport of Li+ ions and suppress dendrite formation.
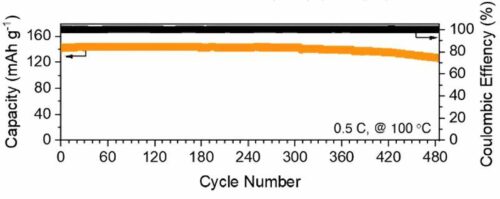
Consequently, these anionic network polymer membranes enable lithium metal batteries to function as safe, long-cycling energy storage devices at high temperatures, maintaining 92.7% capacity retention and averaging 99.867% coulombic efficiency over 450 cycles at 100°C. In contrast, conventional liquid electrolyte Li metal batteries typically perform fewer than 10 cycles at high temperatures. The team potentially paves the way for future advancements in anionic polymer electrolyte design for next-generation lithium batteries. They mention that the innovation opens doors for new battery chemistries that can revolutionise rechargeable batteries for high-temperature applications, emphasising safety and longevity. Apart from applications in high-temperature scenarios, microcrack-free electrolyte membranes also have the potential to enable fast charging due to low overpotential. This capability could allow electric vehicles to recharge when it takes to drink a cup of coffee, marking a significant advancement towards a clean energy future.”






