Penn State’s new lithium extraction method uses electric current and hydrogen peroxide, reducing costs by 35.6% and emissions by 75%.
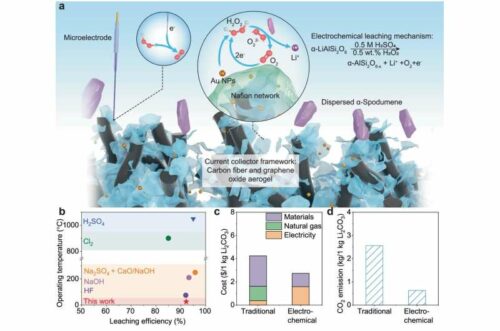
Researchers at Penn State have developed a more efficient method for extracting lithium directly from spodumene ore using an electric current and hydrogen peroxide. This new process could reduce extraction costs by 35.6% and cut CO2 emissions by 75.3%, compared to traditional methods.
Lithium is typically extracted in two ways: from large brine lakes or from ore within rock formations. While 70% of lithium is currently extracted from brines due to lower costs, both methods have significant environmental impacts, according to the researchers.
The brine extraction method can take months, requiring the evaporation of large lakes of concentrated salt solution and the chemical separation of lithium from sodium. This process leaves behind barren soil, unable to support plant life, according to the researchers.
The other common method involves leaching lithium from ore using strong acids or bases at temperatures up to 1,100°C. At these high temperatures, spodumene’s atomic density is reduced, allowing acid to replace lithium ions with hydrogen ions, freeing the lithium for extraction. However, this process is energy-intensive and requires concentrated acids, increasing both costs and safety risks.
The research team’s innovative method uses an electrical field to electrochemically leach lithium from the mineral, converting the solid-state resource into a soluble liquid. Unlike traditional leaching methods, this approach doesn’t require high temperatures, pressure, or concentrated agents to alter the mineral’s natural state.
In initial trials, the electric current excited electrons in the mineral, releasing some lithium ions, though not enough for large-scale commercial use. Shi suggested adding hydrogen peroxide to enhance the leaching process, reducing barriers and improving electron transport for more efficient extraction.
In their studies, the researchers achieved 92.2% efficiency, similar to traditional methods. However, their approach requires less processing time, as the mediator doesn’t introduce impurities that would need further separation.






