For the first time, observing lithium-ion flow through a battery interface may aid in optimising material design.
Courtesy of the researchers
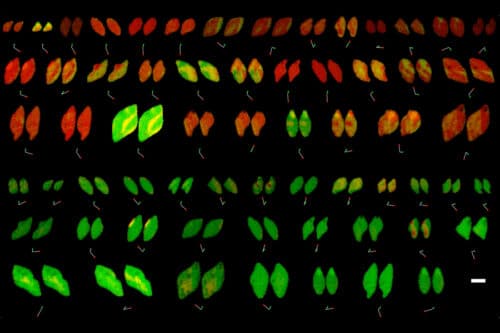
Exploring the world of battery nanoparticles through captivating X-ray movies has been an exciting endeavour. However, comprehending the nuanced intricacies of their functionality remained a challenge due to the overwhelming richness of information contained in these movies.
Researchers at MIT, Stanford University, SLAC National Accelerator, and the Toyota Research Institute have made significant discoveries about the reactivity of lithium iron phosphate, a crucial material used in electric car batteries and other rechargeable energy storage systems, by analysing X-ray images. This approach can unveil insights into various materials, including batteries and biological procedures like embryo cell division.
Modelling Reaction Rates
By examining X-ray images of 63 lithium iron phosphate particles during charging and discharging, the researchers discovered that the movement of lithium ions within the material closely resembled Bazant’s earlier computer simulations. They trained a computational model using all 180,000 pixels as data points to develop equations accurately describing the battery material’s non-equilibrium thermodynamics and reaction kinetics. Furthermore, they noted that observed lithium-ion flow patterns could indicate spatial variations in the rate of lithium-ion absorption at different locations on the particle’s surface. Additionally, researchers linked reaction rate differences to carbon coating thickness on lithium iron phosphate particles. This coating enhances conductivity, which is crucial for battery performance.
Optimised Materials
The study’s findings suggest that fine-tuning the carbon layer thickness on the electrode surface could enhance battery efficiency. The researchers emphasise the importance of controlling reaction kinetics at the electrolyte-electrode interface in battery optimisation and design. They view this publication as the culmination of six years of dedicated collaboration, enabling a deeper understanding of battery inner workings and aiming to enhance future battery design with this newfound knowledge. Beyond battery materials, the researchers anticipate applying this analysis to study pattern formation in various chemical and biological systems.





