The protective layer extends the lifespan of photoelectrodes in solar hydrogen production, improving stability and efficiency for over 400 hours of water splitting reactions.
The commercialization of solar-driven green hydrogen production is progressing, thanks to the development of a protective material that extends the lifespan of photoelectrodes—the components of this technology. Researchers from the School of Energy and Chemical Engineering at UNIST, in collaboration with researchers from the University of Zurich (UZH), have engineered a protective layer that improves the durability of metal oxide-based photoelectrodes used in solar hydrogen production.
Photovoltaic hydrogen production uses sunlight to produce hydrogen through the electrochemical splitting of water. The process depends on a photoelectrode, which absorbs solar energy to drive reactions that break down water into hydrogen and oxygen. When illuminated by sunlight, the photoelectrode triggers electrochemical reactions that separate water molecules, producing hydrogen gas.
One of the challenges in this technology is the corrosion of photoelectrodes during water oxidation, making the need for protective materials crucial for commercialization. Although metal oxide-based photoelectrodes are cost-effective, their development has been hindered by the lack of suitable protective coatings.
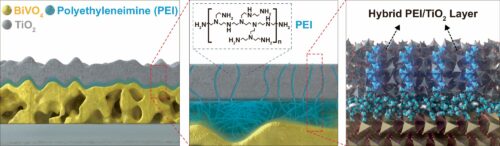
To address this issue, the research team has integrated polyethyleneimine (PEI) polymer with titanium dioxide (TiO2), a material traditionally used to protect semiconductor photoelectrodes. This protective layer blocks electrons—negatively charged particles generated by light absorption—while selectively permitting holes—positively charged particles—to drive water oxidation reactions. This control enhances the performance of photoanodes and prevents corrosion.
When applied to BiVO4 photoanodes, the protective layer allowed stable water splitting reactions for over 400 hours at a current density of 2.03 mA/cm². This is an improvement in stability compared to photoelectrodes without protective layers, which typically degrade within five hours. Current density is an indicator of photoelectrode efficiency. Additionally, this protective layer can be used with a variety of metal oxide-based photoelectrodes, including iron oxide (Fe2O3).
Reference: Sanghyun Bae et al, A hole-selective hybrid TiO2 layer for stable and low-cost photoanodes in solar water oxidation, Nature Communications (2024). DOI: 10.1038/s41467-024-53754-9








