Researchers from the Chinese Academy of Sciences have introduced contact-electro-catalysis, harnessing electron transfer to recycle LIBs efficiently and sustainably. Learn how this eco-friendly approach could reshape the future of battery recycling and support the global drive towards carbon neutrality.
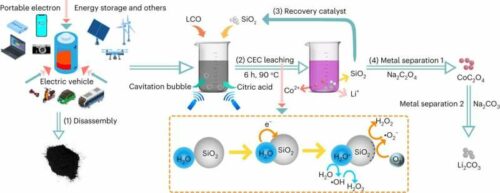
Lithium-ion batteries (LIBs), the powerhouse behind most of today’s electronic devices, have long been celebrated for their versatility, durability, and compatibility with modern manufacturing processes. These rechargeable wonders have been pivotal in powering tech-driven lives while aligning with global efforts to achieve carbon neutrality. The sustainable recycling of LIBs has remained a challenging frontier within the energy sector. Existing methods have often proven ineffective, expensive, or harmful to the environment, hampering the efforts to make LIBs more eco-friendly. LIBs rely on materials like cobalt and lithium, which are dwindling in availability on Earth. Sustainable ways to extract and recycle these materials from spent batteries are critical to meeting the growing demand for LIBs.
Researchers from the Chinese Academy of Sciences have unveiled a solution that leverages the electron transfer occurring during liquid-solid contact electrification to generate free radicals that initiate crucial chemical reactions. The researchers emphasized the growing demand for LIBs amid the global push for carbon neutrality and the pressing need to enhance current recycling methods regarding environmental impact, cost, and efficiency. Their “mechano-catalytic” approach, contact-electro-catalysis, utilises radicals produced through contact electrification to facilitate metal leaching under ultrasonic waves, with recyclable SiO2 catalysing the process.
To assess the method’s viability, the team embarked on a study to replace traditional chemical agents used in LIB recycling. They achieved continuous solid-liquid contact and separation through cavitation bubbles under ultrasound waves, resulting in the constant generation of reactive oxygen. Their approach yielded results for lithium and cobalt recycling in worn-out LIBs. For lithium cobalt (III) oxide batteries, the leaching efficiency reached 100% for lithium and 92.19% for cobalt at 90°C within six hours. For ternary lithium batteries, the leaching efficiencies of lithium, nickel, manganese, and cobalt reached 94.56%, 96.62%, 96.54%, and 98.39% at 70°C, respectively, within six hours.
These initial tests showcased the method’s potential for achieving low-cost, sustainable, and large-scale recycling of the valuable materials found within LIBs. Future research endeavours will further refine this approach, evaluate its strengths and limitations, and pave the way for its practical implementation in real-world scenarios. The quest for eco-friendly and cost-effective LIB recycling may have just found a powerful ally in contact-electro-catalysis, promising a greener and more sustainable future for the electronics industry.