Transforming CO2 into propane using tri-molybdenum phosphide, pioneering a sustainable carbon cycle and renewable energy pathway.
Over the past three centuries, human actions, particularly since the Industrial Revolution, have dramatically raised greenhouse gas concentrations in the atmosphere. Fossil fuel use, industrial activities, deforestation, and waste handling are the primary contributors.
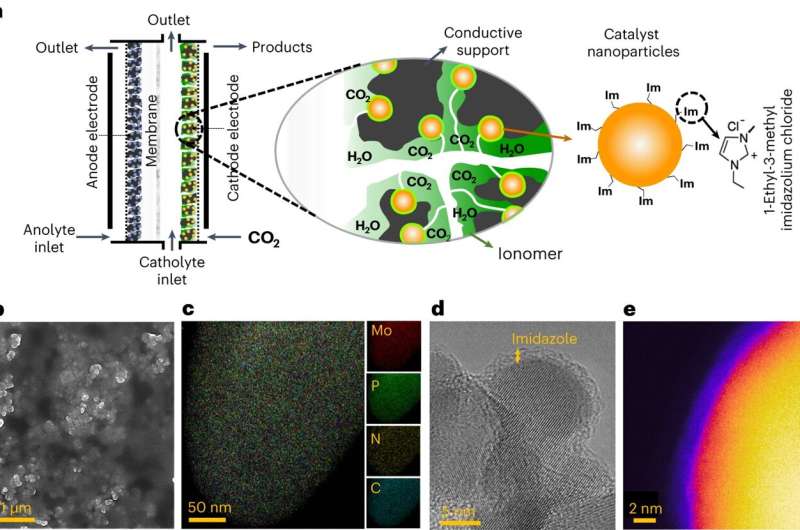
Researchers from the University of Pennsylvania, Illinois Institute of Technology, and the University of Illinois at Chicago have devised a method to transform CO2 emissions into propane, a cleaner and more potent fuel. This electrochemical CO2 conversion offers a path for renewable energy storage and closing the human-made carbon cycle. This study opens doors for innovative energy storage solutions and significant CO2 reduction.
Producing energy-rich multi-carbon products like C3H8 is challenging due to numerous intermediates in chemical conversion. Most methods to enhance selectivity for these molecules are energy-intensive. Researchers have favoured copper for turning CO2 into valuable chemicals and fuels. This has mostly yielded low-energy density fuels like methane. The team explored alternatives to copper, aiming for better selectivity for multi-carbon products. Their research led them to integrate ionic liquid (IL) into the system, ultimately focusing on tri-molybdenum phosphide (Mo3P) as the catalyst. The team also notes that this key finding led to a new paradigm for exploring the relationship between materials in electrocatalytic systems.
The U.S. targets a 50-52% reduction in greenhouse gas emissions from 2005 by 2030, supporting the global net-zero by 2050 goal. With power and industry sectors being major CO2 contributors, renewable chemical manufacturing is vital to close the carbon cycle sustainably.
The researchers have two future goals: First, they aim to catalogue ionic liquids and their efficacy in fuel-related catalysts and other electrochemical systems. Second, they will explore new catalysts to transform CO2 into fuels ranging from gas to light oil. By converting CO2 into natural gas, propane, or even jet fuel, we can continually reuse carbon atoms, reducing atmospheric release.