Researchers have developed a battery model which increases the storage capacity and lifetime of li-ion batteries.
Lithium-ion batteries have come a long way since they were first introduced. They have some familiar drawbacks as well, such as short lifetimes, overheating and supply chain challenges for certain raw materials. They are used in every other handy device you use. But the short lifetime of these batteries also affect the life of the respective device which is not considerable over time.
Researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory has found its solutions by testing new materials in battery construction. One such material is sulfur. Sulfur is extremely abundant and cost effective and can hold more energy than traditional ion-based batteries.
This battery design pairs a sulfur-containing positive electrode (cathode) with a lithium metal negative electrode (anode). In between those components is the electrolyte, or the substance that allows ions to pass between the two ends of the battery.
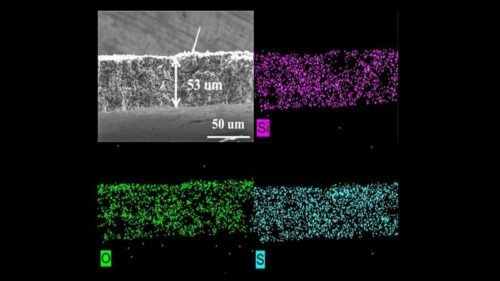
Current battery technology consists of a dense, heavy protective interlayer which reduces the battery storage capacity per unit weight for the battery. To address this, researchers developed and tested a porous sulfur-containing interlayer. Tests in the laboratory showed initial capacity about three times higher in Li-S cells with this active, as opposed to inactive, interlayer. More impressively, the cells with the active interlayer maintained high capacity over 700 charge-discharge cycles. Researchers conducted experiments at the 17-BM beamline of Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility to further study the redox-active layer.
The data gathered from exposing cells with this layer to X-ray beams allowed the team to ascertain the interlayer’s benefits. The data confirmed that a redox-active interlayer can reduce shuttling, reduce detrimental reactions within the battery and increase the battery’s capacity to hold more charge and last for more cycles. These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development. Researchers believe this technology can be shortly adopted in everyday use.






