Researchers have dived into the battery’s molecular space to enhance the performance as well the battery age.
Electronics is a study of movement of electrons in semiconductor and conductor. Every electronic device from a tiny microchip to a supercomputer, functions due to these micro particles. Considering a battery in this case, the charging and discharging of ions is what drives the whole concept. After a few hundred charge cycles, you’ll notice—your phone, laptop or electric car battery wears out more quickly. Eventually, it stops holding a charge at all.
Researchers at the University of Chicago’s Pritzker School of Molecular Engineering (PME) have now used a combination of high-powered electron microscopy and computational modeling to understand, at an atomic level, exactly what occurs when lithium-ion batteries degrade. Their research points toward one approach to designing longer-lasting lithium-ion batteries—by focusing on an oft-ignored structural component, the carbon binder domain (CBD).
When a lithium-ion battery is charged, lithium ions move from a positively charged cathode to a negatively charged anode. To release energy, those ions flow back from the anode to the cathode. Throughout charging cycles, the active materials of the cathode and anode expand and contract, accumulating “particle cracks” and other physical damage. Over time, this makes lithium-ion batteries work less well.
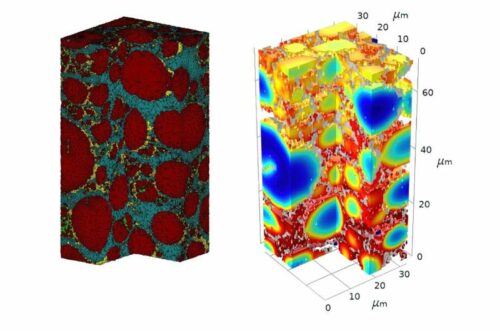
The kinetics of a thick electrode are quite different from those of a thin electrode. Degradation is actually much worse in thicker, higher-energy electrodes, which has been a struggle for the field. Researchers believe it’s also harder to quantitatively study thick electrodes. They used Plasma focused ion beam-scanning electron microscopy (PFIB-SEM) to collect data on a brand new cathode as well as one that had been charged and depleted 15 times. With the data from the electron microscopy experiments, the team built computational models illustrating the process of degradation in the batteries.

The researchers discovered that variation between areas of the battery encouraged many of the structural changes. Electrolyte corrosion occurred more frequently with a thin layer at the surface of the cathode. This top layer therefore developed a thicker resistive layer, which led the bottom layer to expand and contract more than other parts of the cathode, leading to faster degradation.
With their model of the cathode, Meng’s group studied how tweaks to the electrode design might impact its degradation. They showed that changing the CBD structure network could help prevent the worsening of contacts between the CBD and active materials, making batteries last longer—a hypothesis that engineers can now follow up with physical experiments.
Reference : Minghao Zhang et al, Coupling of multiscale imaging analysis and computational modeling for understanding thick cathode degradation mechanisms, Joule (2022). DOI: 10.1016/j.joule.2022.12.001









