Researchers from Japan have developed a laboratory data management platform that enables automated analysis and lossless sharing of material exploration data in the form of knowledge graphs in electronics laboratory books
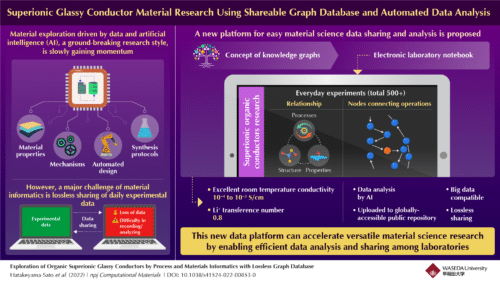
The scientific community provides the materials and research papers mainly emphasized structure-property relations and hardly focus on essential experimental protocols. The back-and-forth sharing of data within the scientific community results in the loss of data. To overcome these issues, a team of researchers led by Assistant Professor Kan Hatakeyama-Sato and Professor Kenichi Oyaizu from Waseda University in Japan have invented a lossless laboratory data management platform that details the relations between properties, structures, and experimental processes in electronic laboratory notebooks.
Hatakeyama-Sato, Assistant Professor, from Waseda University in Japan remarks, “This platform is currently applicable to solid-state batteries and with improved performance will be able to contribute to the development of safer and high-capacity batteries.”
The electronic laboratory notebook includes all experimental events and related environmental parameters which are represented as knowledge graphs. The team implemented an AI-based algorithm that could automatically convert these knowledge graphs into tables and upload them into a public repository. This process ensured that data sharing was lossless because of which the scientific community will gain better insights into the experimental conditions.
They experimented with their platform to explore superionic conductivity in organic lithium (Li+)-ion electrolytes. They noted everyday raw data which included both successful and unsuccessful ones from over 500 experiments in the electronic laboratory notebook. Next, the data conversion module automatically transformed the knowledge graph data into machine-learnable datasets and analyzed the relationship between experimental operations and results. This analysis showed the important parameters needed to achieve excellent room temperature ionic conductivity of 10−4–10−3 S/cm and a Li+ transference number as high as 0.8
Discussing its long-term implications, Hatakeyama-Sato further adds, “By sharing raw experimental data among researchers across the globe, novel functional materials could be discovered more quickly. This approach can also accelerate the development of energy-related devices, including next-generation batteries and solar cells.”
Click here for the Published Research Paper






