Researchers from the New Jersey Institute of Technology (NJIT) have developed an electrochemistry-based approach that could allow for instant safety and quality testing of promising biotherapeutics
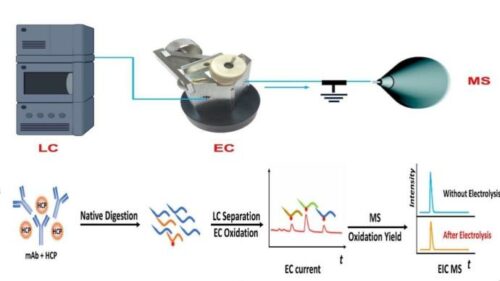
The conventional method for sample testing of biomaterials is expensive and time-consuming. Traditional protein quantification includes the preparation of synthetic isotope-labeled peptides that are used as internal standards to measure total protein concentrations in a sample, this helps researchers actively monitor the efficacy and safety of therapeutic protein components throughout the drug development process. To overcome these hurdles, researchers from NJIT launched a new lab technique that involves a coulometric mass spectrometry (CMS) approach for the complete quantification of proteins without the use of standards.
“This method we’ve developed at NJIT has the potential to have a major impact in quantitative proteomics, and it represents a paradigm shift in the pharmaceutical industry in terms of monitoring biopharmaceutical products and process impurities for quality control,” said Hao Chen, the paper’s corresponding author and professor at NJIT’s Department of Chemistry and Environmental Sciences.
The method applies liquid chromatography-mass spectrometry and an electrochemical flow cell to rapidly quantify and detect changes in target proteins or peptides based on electrochemical signatures. The team demonstrated the method’s capabilities for detecting protein deamidation, it’s a common degradation event in therapeutic proteins resulting from physical or chemical stresses throughout the manufacturing process and storage. They were able to successfully quantify several protein degradation products, including a key intermediate of protein degradation (the formation of succinimide) which has never been done before with absolute quantification due to a lack of standards, according to the study’s authors.
“Such an apparatus allows us to separate peptides after protein digestion with liquid chromatography, monitor peptide oxidation in the electrochemical flow cell to produce an electric current, and measure the oxidation yield with mass spectrometry,” explained the paper’s first author and NJIT Ph.D. student Yongling Ai. “The combination of electric current signals along with the oxidation yield provides sufficient information for absolute quantitation of peptides and proteins.”
“As proteins perform a vast array of functions within organisms, the importance of absolute protein quantitation is hard to overstate,” said Chen. “CMS should speed up processes for disease diagnosis, drug discovery, and development, and it now opens a new door for biologists and biochemists to explore quantities of proteins in the human body that may serve important biological functions or roles as disease biomarkers and drug targets.”
Now, Chen’s lab aims to apply their new method for large scale quantitation of thousands of proteins at once. They also plan to enhance the sensitivity of their CMS analysis to enable quantifying very low levels of proteins in complex biological samples.
Click here for the Published Research Paper