Safety, accuracy and reliability of operation are critical for electro-medical equipment. This article discusses the latest solutions that help install, calibrate and maintain these equipment
Dilin Anand
The most significant advances in the field of medicine have been in the technologies used to diagnose and treat patient. Biomedical equipment used in hospitals include numerous different types of electrodes, transducers, biomedical recorders and patient monitoring systems. We have come such a long way that instruments shown in sci-fi videos like Tricorders are now almost here. In fact, Qualcomm has launched a 10-million dollar competition called Qualcomm Tricorder X to motivate designers. Our point here is that as medical equipment advance, so should the test equipment that help design them.
Test equipment for medical electronics are usually used to calibrate, regulate, fine-tune or test electrical or electronic equipment that are used in a healthcare environment. Apart from use while designing the equipment, these can also be used for servicing, repair and other maintenance tasks.
Major differentiating factors for testing medical electronics
Since treatment given to the patients depends on the measurements by electro-medical instruments, calibrating and later maintaining this precision are of prime importance. Starting from the large X-ray equipment to the smaller patient monitors, all electronic equipment in a hospital need to be maintained as these work within extremely stringent conditions. In addition, electronic wheel-chairs, hearing-aids and surgical equipment are also expected to adhere to strict electro-medical requirements so that these do not interfere with other equipment. Safety, accuracy and reliability of operation are the critical factors.
“Medical device development is a complex process with quality being the highest concern. However, reducing development time is a pressure critical to establishing an early position in a very competitive market. To manage the quality concerns, agencies such as the Food and Drug Administration (FDA) have been established to help guide and enforce best practices for the development of safe and reliable devices. The rest is up to the engineers, to work within those constraints and meet all of the requirements in the most timely and cost-effective manner,” explains Satish Mohanram, technical marketing manager, National Instruments.
Electro-medical test equipment also need to adhere to standards like IEC 62353. The IEC 62353 is a standard for in-service and after-repair testing of medical electronic devices.
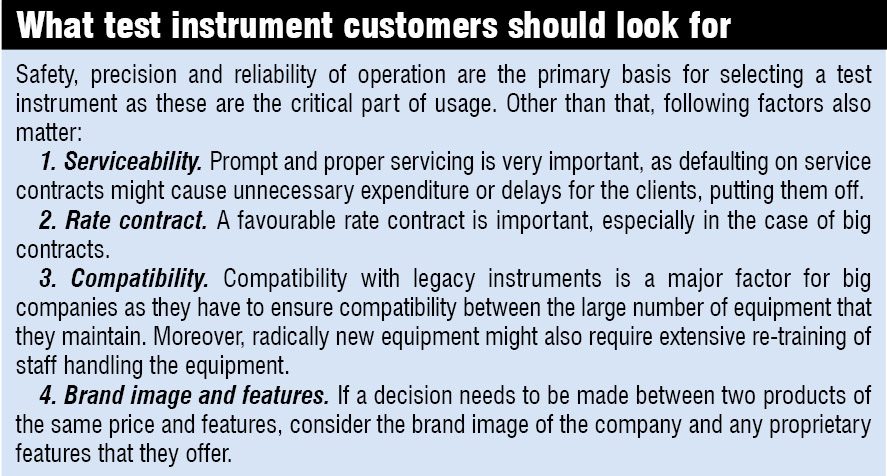
In the box on the next page, we have listed key factors that customers should look at when purchasing test equipment. Apart from those, validation and verification is a very important part of building a measurement and automation solution in the life sciences arena.
Measuring biological electrical phenomena with signal measurement systems is challenging. Electrical signals from the body are tiny microvolt pulses that signal a muscle response or a ‘firing’ neuron. Apart from being very small, these signals are also intermittent and hidden amongst other noise. This necessitates the need for a wide dynamic range, high-quality signal conditioning and storage capacity to store signals for a long duration with precision.
Boosting reliability and efficiency
Electro-medical test systems have been mostly custom-built rack-and-stack test benches, which are slowly becoming software-driven. Concepts such as hardware-in-loop and model-in-loop testing are gaining popularity as these approaches enable thorough testing of the medical equipment’s functionality. Tools such as LabVIEW and reconfigurable input/output hardware play a vital role in these closed-loop complex plant simulation and test scenario implementation.
Any test system that is used in the manufacturing and/or verification of a medical device must also be included in the validation process of that device.
“This means that the test code is subject to the same scrutiny with regards to development practices, change management and documentation as the firmware of the device. This brings a new constraint on the people designing their systems. They have the job of writing and building the test system. The PXI platform with LabVIEW software has been widely used as the test bench for verification and validation, and test cases are reused on the manufacturing line for product end-of-line testing,” explains Mohanram.
Improvements in minimally invasive techniques as well as drug delivery technologies have resulted in advances in imaging and navigation technologies.
“This will continue to improve diagnostic accuracy and also enhance surgical capabilities. Upcoming solutions should also incorporate bioinformatics, which can result in early and faster diagnosis, better prognosis and tailored therapies. Personalisation of such devices could help a lot, where individuals’ medical history and information could be available easily and at one place whenever needed,” adds T. Anand, managing director, Knewron.