The innovation has become an essential part in the way we use portable electronic devices in our daily lives. It has also made possible the creation of new electronic technology.

In an exciting news for the entire electronics community, the Royal Swedish Academy of Sciences has conferred the Nobel Prize in Chemistry 2019 to John B. Goodenough, the University of Texas at Austin, USA, M. Stanley Whittingham, the State University of New York at Binghamton, USA and Akira Yoshino, Asahi Kasei Corporation, Tokyo, Japan and Meijo University, Nagoya, Japan for the development of lithium-ion batteries.
Lithium-ion batteries have become a critical part of electronic devices without which it would have become extremely difficult to power portable electronics that we daily use such as smartphones, laptops and music players. Even electric vehicles are a huge beneficiary of this innovation. Lithium-ion batteries have also enabled the storage of energy from renewable sources, such as solar and wind energy.
How it all started
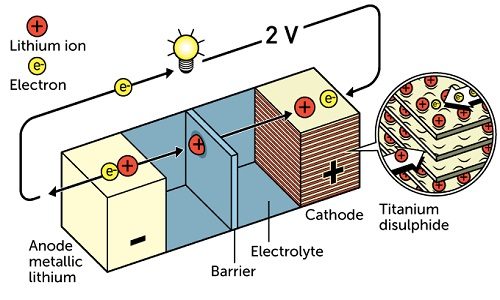
It all began way back in the 1970s when Stanley Whittingham was working on developing a technology that would prove be a better and cleaner alternative to fossil fuels. While doing research on superconductors, he created a battery whose cathode was made from titanium disulphide and the anode was partially made from metallic lithium. This battery literally had great potential, just over two volts. However, due to the its explosive nature, the battery was not considered viable.
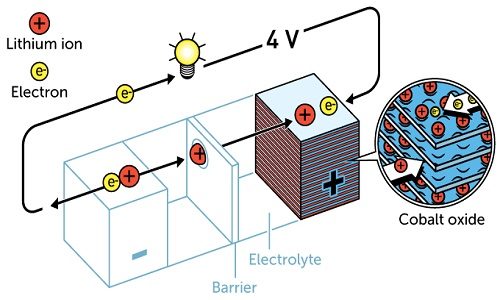
After much research, in 1980, John Goodenough proposed that the previous cathode would have had even greater potential if it was made using a metal oxide instead of a metal sulphide. He demonstrated that cobalt oxide embedded with lithium ions produced as much as four volts. This was an important breakthrough in developing a much more powerful battery.
Taking forward the cathode developed by Goodenough, Akira Yoshino created the first commercially viable lithium-ion battery in 1985. To rectify the battery’s explosive behaviour, he replaced the reactive lithium anode with petroleum coke (a carbon material) anode, which was safely injected with lithium ions.
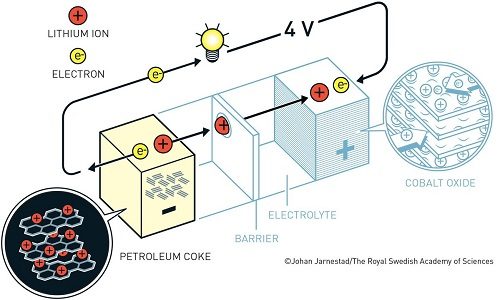
The final result was a robust and lightweight battery that could be charged multiple times. Unlike conventional batteries, lithium-ion batteries were not based upon chemical reactions that broke down the electrodes, but only upon back and forth flow of lithium ions between the anode and cathode.
Present-day battery
The Li-ion battery used in most of the electronic devices these days hasn’t changed much from the previous version, except for some minor developments. The cathode is typically made from a chemical compound called lithium-cobalt oxide (LiCoO2) or from lithium iron phosphate (LiFePO4). The anode is generally made from carbon (graphite) and the electrolyte between them varies from one type of battery to another. During the charging and discharging process, lithium ions flow back and forth between the anode and cathode to produce energy.
These batteries have the advantage that due to high energy density. A typical lithium-ion battery has the capacity to store 150 watt-hours of electricity in 1 kilogram of battery. The can recharged many times and no memory effect, which means that one does not have to completely discharge them before recharging.
Disadvantage is that they are quite sensitive to high temperatures which causes them degrade much faster and can burst into flames when exposed to inflammable material.
Despite the shortcomings, research is going to mitigate the damages. Without any doubt, lithium-ion batteries have revolutionised our lives since they first entered the market in 1991. They have laid the foundation of a wireless, fossil fuel-free society, and are of the greatest benefit to humankind.