Producing light
If we try to run current the other way, with the p-type side connected to the negative end of the circuit and the n-type side connected to the positive end, current will not flow. The negative electrons in the n-type material are attracted to the positive electrode. The positive holes in the p-type material are attracted to the negative electrode. No current flows across the junction because the holes and the electrons are each moving in the wrong direction. The depletion zone increases. The interaction between electrons and holes in this setup has an interesting side effect—it generates light.
Light is a form of energy that can be released by an atom. It is made up of many small, particle-like packets that have energy and momentum but no mass. These particles, called photons, are the most basic units of light. Photons are released as a result of moving electrons. In an atom, electrons move in orbitals around the nucleus. Electrons in different orbitals have different amounts of energy. Generally speaking, electrons with greater energy move in orbitals farther away from the nucleus. For an electron to jump from a lower orbital to a higher orbital, something has to boost its energy level.
Conversely, an electron releases energy when it drops from a higher orbital to a lower one. This energy is released in the form of a photon. A greater energy-drop releases a higher-energy photon, which is characterised by a higher frequency.
In case of LEDs, free electrons moving across a diode can fall into empty holes from the p-type layer. This involves a drop from the conduction band to a lower orbital, so electrons release energy in the form of photons. This happens in any diode, but you can only see the photons when the diode is made of certain material.
Atoms in a standard silicon diode, for example, are arranged in such a way that the electron drops a relatively shorter distance. As a result, the photon’s frequency is so low that it is invisible to the human eye—it is in the infra-red portion of the light spectrum.
This is not necessarily a bad thing. Infra-red LEDs are ideal for remote controls, among other things. Visible light emitting diodes (VLEDs), such as the ones that light up numbers in a digital clock, are made of materials characterised by a wider gap between the conduction band and lower orbitals. The size of the gap determines the frequency of the photon—it determines the colour of the light.
While LEDs are used in everything from remote controls to digital displays on electronics, VLEDs are growing in popularity and use, thanks to their long lifetimes and miniature size. Depending on the materials used in LEDs, these can be built to shine in infra-red, ultra-violet and all colours of the visible spectrum in between.
When current flows across a diode, negative electrons move in one way and the positive holes move in another way. The holes exist at a lower-energy level than the free electrons, so when a free electron falls, it loses energy. This energy is emitted in the form of a light photon. The bigger fall of electron means higher energy or frequency of light emitted.
LED applications
Light emitting diodes are real unsung heroes of the electronics world. These do dozens of different jobs and are found in all kinds of devices. Among other things, these form numbers on digital clocks, transmit information from remote controls, light-up watches and tell you when your appliances are turned-on. Collected together, LEDs can form images on a jumbo television screen or illuminate a traffic light.
Visible LEDs are used in many electronic devices as indicator lamps, in automobiles as rear-window and brake lights, and on billboards and signs as alpha-numeric displays or even full-colour posters.
Infra-red LEDs are employed in autofocus cameras and television remote controls, and also as light sources in fibre-optic telecommunication systems.
Any LED can be used as a light source for a short-range fibre-optic transmission system, that is, over a distance of less than 100 metres (330 feet). For long-range fibre optics, however, the emission properties of the light source are selected to match the transmission properties of the optical fibre, and in this case, infra-red LEDs are a better match than visible LEDs.
Glass optical fibres suffer from lowest transmission losses in the infra-red region at wavelengths of 1.3 to 1.55 micrometres. To match these transmission properties, LEDs are employed that are made of gallium-indium-arsenide-phosphide, layered on a substrate of indium-phosphide. The exact composition of the material may be adjusted to emit energy precisely at 1.3 or 1.55 micrometres.
LED advantages
Basically, LEDs are just tiny light bulbs that fit easily into an electrical circuit. But unlike ordinary incandescent bulbs, these do not have a filament that will burn out, and these do not get especially hot. LEDs are illuminated solely by the movement of electrons in a semiconductor material, and these last just as long as a standard transistor.
The interior of an LED is actually quite simple, which is one of the reasons this technology is so versatile. The lifespan of an LED surpasses the short life of an incandescent bulb by thousands of hours. LEDs also fit more easily into modern electronic circuits.
The main advantage is efficiency as LEDs generate very little heat. A much higher percentage of the electrical power goes directly to generating light, which cuts down on the electricity demands considerably. Tiny LEDs are already replacing tubes that light-up LCD HDTVs to make dramatically-thinner televisions. Though these often come in tiny packages, LEDs produce a large amount of light and are used in an ever-growing list of technologies.
Although LEDs will continue to be widely used as small indicator lamps, the number of applications these can find is increasing as the technology improves. New very-high luminance diodes are now available. LEDs are even being used as a form of illumination, an application which these were previously not able to fulfil because of their low-light output. New colours are being introduced. White and blue LEDs, which were previously very difficult to manufacture, are now available. In view of the on-going technological development, and their convenience of use, these devices will remain in electronics catalogues for years to come.
LED disadvantages
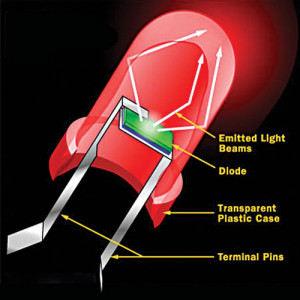
While all diodes release light, most do not do it very effectively. In an ordinary diode, the semiconductor material itself ends up absorbing a lot of the light energy. LEDs are specially constructed to release a large number of photons outward. Most of the light from the diode bounces off the sides of the bulb, travelling on through the rounded end.
Additionally, LEDs are housed in a plastic bulb that concentrates the light in a particular direction. Up until recently, LEDs were too expensive to use for most lighting applications because these are built around advanced semiconductor material. But advantages like energy-efficiency and longer lifespans have made LEDs a more cost-effective lighting option for a wide range of situations, competing with incandescent and compact fluorescents.













