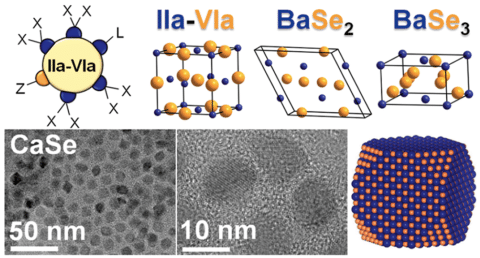
Scientists from Ames National Laboratory and Iowa State University have developed a colloidal synthesis method that would assist in synthesizing alkaline-earth chalcogenides
(AeCh) nanocrystals
The most used semiconductors i.e. lead or cadmium is found to be harmful to humans and the environment. The process used to synthesize these materials involved solid-state reactions, these reactions take place at very high temperatures (above 900 °C or 1652 °F) and require days to weeks to complete the reaction. To overcome these issues, scientists have developed a colloidal synthesis method for alkaline earth chalcogenides as this method involves much lower temperatures (below 300 °C or 572 °F) and has shorter reaction times.
The alkaline-earth chalcogenides are widely available and consist of biocompatible elements which are great alternatives compared to widely used toxic and expensive semiconductors. The alkaline earth chalcogenides can be used for varieties of applications such as bioimaging, LEDs, and thermal sensors. It can be also used to make optical materials such as perovskites, which convert light into energy.
Nanocrystal size is important because it determines the optical properties of some materials. The team got to know that the colloidal synthesis method can assist them in controlling the size of the nanocrystals. Vela, Ames Lab scientist, explained that by altering the size of the particles, scientists can efficiently make the materials absorb light. “This means we can potentially synthesize materials that are more suited for specific applications just by changing the nanocrystal size,” he said.
Vela said that their research fills a need to improve scientists’ understanding of photovoltaic, luminescent, and thermoelectric materials that are made of earth-abundant and non-toxic elements. He said, “We hope that our developments with this project ultimately aid in the synthesis of more complex nanomaterials, such as the alkaline-earth chalcogenide perovskites.”
Click here for the Published Research Paper








