Li-ion cell is one of the most important energy storage devices in today’s time. The cell has a lot of different chemistries, and the properties of these cells depend on their internal chemistry. Every single chemistry has its own pros and cons.
Lithium-ion (Li-ion) cells provide one of the most important energy storages today. These cells are used in many electronic items including smartphones, laptops, personal portable equipment, cameras, etc. In fact, Li-ion cells have paved the way for efficient and long ranged electromobility, thus making electrical vehicles (EVs) practical for our daily commute.
Li-ion cells are secondary cells or, in other words, rechargeable cells, that is, they can be recharged by passing current in the reverse direction. Li-ion batteries are lightweight and have higher energy density than other types of rechargeable cells, thus making them suitable for energy-intensive applications where constant replenishment of energy is essential, such as in our daily electronics gadgets, EVs, home energy storage system. These are used even in applications where a large amount of energy is stored, such as grid energy storage application.
This article describes the six most common Li-ion chemistries and the advantages as well as disadvantages of each type of cell, along with their properties.
Like many other commonly available cells, Li-ion cells are made up of four components—cathode, anode, electrolyte, and separator. Li-ion cells commonly use graphite at anode and an intercalated lithium compound at cathode. The capacity and voltage of a Li-ion cell is dependent on the cathode while the charge and discharge rate of a battery depends on the anode. The amount of lithium and the active material in the cathode dictates the battery capacity and voltage while the potential difference between the anode and the cathode determines the voltage of a battery.
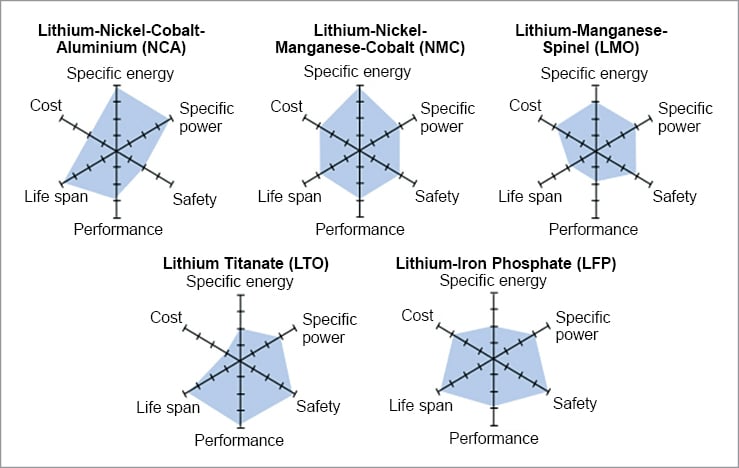
The active compounds in Li-ion batteries interact in complex and complementary ways. Therefore, understanding Li-ion cells’ chemistry is essential for selecting the most suitable one for an application. The six most common Li-ion battery cells are described below.
LCO: Low energy density, high-risk cell
Lithium cobalt oxide (LCO), also known as lithium cobaltate, is a type of Li-ion cell whose cathode is made of lithium, cobalt, and oxygen. LCO cells have a lower energy density of 150 to 180Wh/kg and a nominal voltage of 3.7V. LCO’s main element, cobalt, is an energy-dense material that is also very volatile. Hence, LCO batteries have high specific energy but are not suitable for high charge and discharge rates. Furthermore, these batteries have poor thermal stability, which makes them unsuitable for operation in harsh environments. LCO batteries are sensitive to overcharging and operation in high-temperature environments and are prone to thermal runaway.
Application. LCO batteries are more suitable for use as energy batteries than power batteries and hence are used in such applications as smartphones, cameras, etc.
LMO: Cell with low life and high C-rates
Lithium manganese oxide (LMO) batteries, or lithium-ion manganese batteries, are based on manganese oxide, which is a non-toxic and earth-abundant element, making these batteries economical. These batteries have a nominal voltage of 3.9V and an energy density of 100 to 150Wh/kg. These batteries also have an exceptionally low self-discharge rate. The lower internal resistance and high-temperature stability make these batteries a safer option for power-intensive applications. Moreover, the LMO batteries can achieve high charge and discharge rates due to their lower internal resistance. But LMO cells have a lower energy capacity and a low operational life of 300 to 700 cycles, which has resulted in limited research and advancement of this battery chemistry.
Application. LMO cells are used as power batteries in applications requiring high continuous power and are thus ideal for use in medical, military, and industrial applications. These can also be used in power tools, external defibrillators, etc.
LFP: Long lasting, affordable, and safe cell
Lithium iron phosphate cells, also known as lithium ferro-phosphate (LFP) cells, are one of the most common cell chemistries in today’s time. These cells have a nominal voltage of 3.2V and an energy density of 90 to 160Wh/kg. Although having a lower energy density than many other chemistries, LFP has a very high operational life of 7,000 to 12,000 charge cycles. The LFP cells have high thermal stability, low self-discharge rates, and low internal resistance, which make LFP cells suitable for power operations that can achieve high charge and discharge rates. LFP cells are one of the most affordable chemistries, considering their long operational life.
Application. LFP cells are suitable for high-power applications and can be used in harsh conditions, making them a good option for applications in low-powered EVs, such as e-rikshaws and e-bikes. These are also suitable for industrial equipment that have previously relied on lead-acid batteries, such as forklifts, heavy machines, and other equipment.
| Some important characteristics of different Li-ion cell chemistries | ||||||||||
| Chemistry | Cell Voltage (In volts) |
Energy Density (WH/kg) |
Self- discharge (% / month) |
Temperature range (in °C) | Round trip efficiency (in %) | Cycles Times (maximum) | Cost | Weight | Memory | Safety |
| LCO | 3.7 | 150-180 | 10 | -40 to 80 | 90 | 1000 | Expensive | Light | No | Bad |
| LMO | 3.7 | 100-150 | 1 | -40 to 85 | 95 | 700 | Cheap | Heavy | No | Good |
| LFP | 3.2 | 90-160 | 5 | -40 to 80 | 95 | 12,000 | Cheap | Light | No | Very good |
| NMC | 3.6 | 150-220 | 10 | -40 to 70 | 93 | 2000 | Expensive | Light | No | Bad |
| NCA | 3.6 | 200-260 | 90 | 3000 | Expensive | Light | No | Good | ||
| LTO | 2.4 | 50-80 | 5 | -40 to 55 | 97 | 20,000 | Expensive | Light | No | Very good |
NMC: High-performing, affordable cell
Lithium nickel manganese cobalt oxide (NMC) cells have a cathode made up of a combination of nickel, manganese, and cobalt—commonly in the ratio of 60%, 20%, and 20%, respectively. The properties of the cell can be altered by changing the proportion of each element to achieve either a higher specific energy density or a higher specific power. The nominal voltage of the NMC cell is 3.7V and a high energy density of 150-220Wh/kg. These batteries have a low self-heating rate and are cheaper than most other chemistries, which makes them highly popular.
Application. NMC batteries are used in power tools and are one of the most preferred battery chemistries for EVs.
NCA: High energy and lasting cell
Lithium nickel cobalt aluminium oxide (NCA) cells are basically LCO cells with nickel and aluminium added as extra elements in their cathode. NCA cells have a high energy density of 200 to 260Wh/kg and a nominal voltage of 3.6V. The cells have a decent lifespan of 2000 charge cycles. Although the high proportion of nickel in the cathode improves the energy density of the cell, these cells are not stable and therefore require additional safety requirements to monitor their behaviour and keep users safe. NCA batteries can sustain a high charging current, thus enabling fast charging, but the limited availability of cobalt and nickel makes these batteries expensive.
Application. The NCA batteries have a high energy density, making them suitable for use in electric powertrains, grid energy storage, etc.
LTO: Long-lasting but low energy density cell
Lithium titanate (LTO), also known as li-titanate, cells incorporate advanced nanotechnology in their anode. Unlike other cells, which employ graphite, LTO’s anode is made up of lithium titanate, a highly porous material that has up to 33x more surface area per gram than carbon. The high surface area allows faster charge and discharge rates. The LTO cells have a poor energy density of around 50 to 80 Wh/kg and has a nominal voltage of 2.4V. The high surface area enables faster movement of electrons, which enables discharge rates exceeding 30C for short period of time. (The charge and discharge current of a battery is measured in C-rate. Most portable batteries are rated at 1C.) LTO is one of the safest chemistries available in the market. These batteries have a very high operation life of 7,000 to 20,000 cycles.
Application. LTO batteries have been employed in the Japanese-only variant of Mitsubishi’s i-MiEV and in some Honda bikes and cars. The LTO cells are not commonly used in consumer electronics due to their lower energy density, but their fast charging and discharging rate make them suitable for applications in heavy machinery, power tools, EVs, etc. In future, they may find application in storing wind and solar energy and creating smart grids.
Comparison
Li-ion cells have very different characteristics based on their internal construction. The table above compares some important characteristics of different Li-ion cell chemistries for reference.
Li-ion cell is one of the most important energy storage devices in today’s time. The cell has a lot of different chemistries, and the properties of these cells depend on internal chemistry of the cells. Every single chemistry has its own pros and cons. Therefore, selection of the correct cell is crucial for achieving the best cost, performance, and weight of a product.
The author, Sharad Bhowmick, works as a Technology Journalist at EFY. He is passionate about power electronics and energy storage technologies. He wants to help achieve the goal of a carbon neutral world.









Indepth analysis.
The author, Sharad Bhowmick, has done a very broad coverage with more than enough data on this issue in his article, “Six Most Important Lithium-Ion Battery Chemistries”.
Very good work. So informative that even a novice can grasp the essentials while designing Electronic Devices.
Congratulations! And thanks a lot.