Wearable optical heart-rate monitors have become quite popular as people strive to be more proactive about managing their health. Resting heart rate, after all, provides a good indicator of one’s well-being. These monitors commonly rely on photo plethysmography (PPG), an optical measurement of the volumetric change of blood in tissue as a result of the cardiac cycle. To serve as the light source, LEDs are typically used on the transmit path. On the receive path, you’ll find photodiodes that collect the light that refracts and reflects off of the blood flow. Algorithms are used to generate pulse readings.
Skin is a complex, heterogeneous matrix of constituents that both scatter and absorb light. This complexity brings challenges to developing wearable devices that accurately measure heart rate from a fingertip. Modeling skin involves stratifying the tissue matrix into multiple layers. At each stratified layer, we can apply absorption, scattering, anisotropy, and refractive index properties representing the bulk behavior at each layer.
Optical design software analyzes the stratified tissue model along with the optical model for the optical heart-rate monitor. The software traces the path of each ray emitted by the LED. You can change system parameters to influence path length and maximize the receive path signal. For instance, changing the wavelength of the source can affect the depth of penetration. Ultimately, what you want to do is interrogate a desired depth in the skin – for example, the blood dermis layer for improved optical heart-rate monitoring.
Any differences in the optical properties of skin will influence the magnitude and quality of the PPG signal detected. Fortunately, you can improve performance through informed device design.
Measuring PPG Signals
Measuring PPG signals with a wearable device is challenging due to factors such as ambient light cancellation, signal-to-noise ratio, motion compensation, and power consumption. For signal chain optimization, heart-rate monitoring algorithms typically require a signal-to-noise ratio of more than 10dB.
Looking at a traditional receiver path, what you’ll find is that a transimpedance amplifier (TIA) converts the photo current to a voltage. Depending on the photodiode area and quantum efficiency (QE), the photo current is typically in sub-nanoamps to tens of microamps. Unless well managed, ambient light and cross-talk can be greater than the signal. Good designs will be shot-noise limited.
Maxim’s receive path, on the other hand, uses a current-mode, continuous-time, sigma-delta converter to convert the current directly from the photodiode. There are various benefits to this type of architecture:
• Wide ambient cancellation range, limited by VDD and the size of P1 in Figure 1
• Wide signal range, independent of VDD
• Area efficiency, avoiding large anti-aliasing filters
• Power efficiency, avoiding the additional current of a TIA
• High signal-to-noise ratio, close to the theoretical maximum
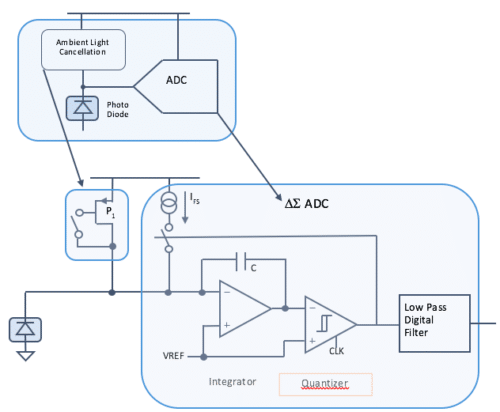
Figure 1: Continuous-time, current-mode, sigma-delta ADC
Considering ambient light cancellation, both DC and AC ambient light rejection are important. DC ambient light can saturate the sensor. AC ambient light can make signal detection difficult. Narrow pulses are more effective than wide pulses. However, noise can be a challenge because of the high bandwidth needed.
For a more effective ambient light cancellation, Maxim applies a two-step approach (depicted in Figure 2):
• Step 1: Analog course cancellation, where the LED is off and ambient light is sampled on the gate of P1
• Step 2: Digital fine cancellation, where the LED is off and a digital filter removes residual DC, AC, and 1/F noise
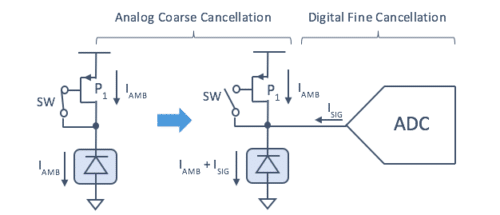
Figure 2: Two-step approach for effective ambient light cancellation.
The transmit path of a heart-rate monitor (Figure 3) consists of the LED voltage (VLED), which must be high enough to support the forward voltage (VF) of the LED; an operational amplifier (op amp), which must have good linearity and a high power supply rejection ratio (PSRR) to mitigate supply noise; and an LED, which is typically green for most heart-rate monitors.
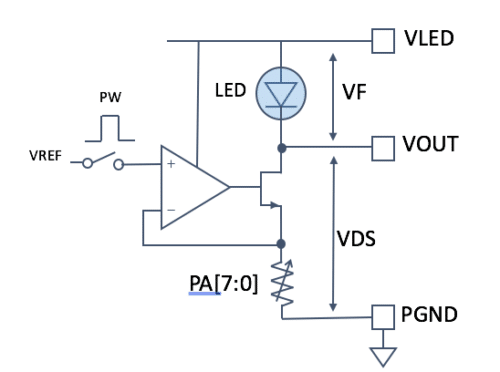
Figure 3: Transmit path for heart-rate monitoring
When you’re developing heart-rate monitoring systems, you must also consider the algorithms involved. Noise rejection, signal detection, and motion compensation are all important challenges to address. For example, to save power, the device must be able to automatically detect when it comes into contact with human skin. And for accuracy, the device must be able to reject ambient and analog front-end (AFE) noise.
Accurate detection of PPG peaks (also known as systolic peaks) and PPG troughs is essential for heart-rate and blood-oxygen monitoring. In traditional algorithms, motion compensation uses fast Fourier transforms (FFTs) or other classical signal-processing methods. While implementing this approach is relatively simple, it is almost impossible to eliminate all motion types with classical filters.
After motion artifacts are removed, heart-rate determination has traditionally used spectrum analysis, polynomial fit, or standalone Kalman filters. However, these methods also have drawbacks:
• An adequate number of heartbeats is required for a decent FFT or polynomial fit
• FFT always has a “fuzzy” peak at the heart-rate frequency because of respiratory sinus arrhythmia, which makes it hard to know what frequency to report
• Kalman models cannot be an exact model for real-life PPG patterns
• It’s hard to separate motion from heart rate with spectrum analysis because the frequencies are close
To overcome many of these shortcomings, Maxim provides a proven and extensively tested proprietary algorithm.
Advancing Designs for Wearable Health
Aside from addressing the requirements for measuring PPG signals, wearable heart-rate monitoring devices must also meet unique design parameters. Power management/long battery life, ultra-small form factor, clinical performance, integration, and low-power operation are all key considerations.
There is a variety of wrist-based wearable PPG sensor designs on the market. However, we’re all still looking out for the optimal wrist-based heart-rate monitor design. As you plan your next design, consider the resources available to help you meet the technical and time-to-market challenges. For example, Maxim’s hSensor Platform can help you quickly and easily evaluate customer health applications and trim down your production development time by up to six months. The hSensor Platform includes a temperature sensor, biopotential (ECG) AFE, pulse oximeter and heart-rate sensor, integrated power management IC (PMIC), and an Arm Cortex-M4F MCU for wearables. Find out more about Maxim’s various wearable health solutions, and challenge yourself to create the ideal design for wrist-based heart-rate monitors.






